5-year-old with Duchenne muscular dystrophy receives first-ever FDA-approved gene therapy
This treatment has the potential to change the lives of thousands of children diagnosed with DMD, a debilitating neuromuscular disease.
[Aug. 12, 2023: Staff Writer, The Brighter Side of News]
Duchenne muscular dystrophy (DMD) is a severe genetic disorder that leads to progressive muscle weakness. (CREDIT: Creative Commons)
In a significant leap for neuromuscular medicine, the Abigail Wexner Research Institute at Nationwide Children’s Hospital has unveiled a recently approved gene therapy for Duchenne muscular dystrophy (DMD). This transformational treatment has the potential to change the lives of thousands of children diagnosed with DMD, a debilitating neuromuscular disorder.
Duchenne muscular dystrophy is a particularly severe muscular disorder caused by a mutation in the DMD gene. This mutation prevents the production of the essential dystrophin protein, leading to a rapid decline in muscle strength and mass.
The disorder primarily affects boys and is marked by progressive symptoms. Over time, these children struggle with deteriorating motor skills, facing increasing challenges in walking, breathing, and maintaining heart function.
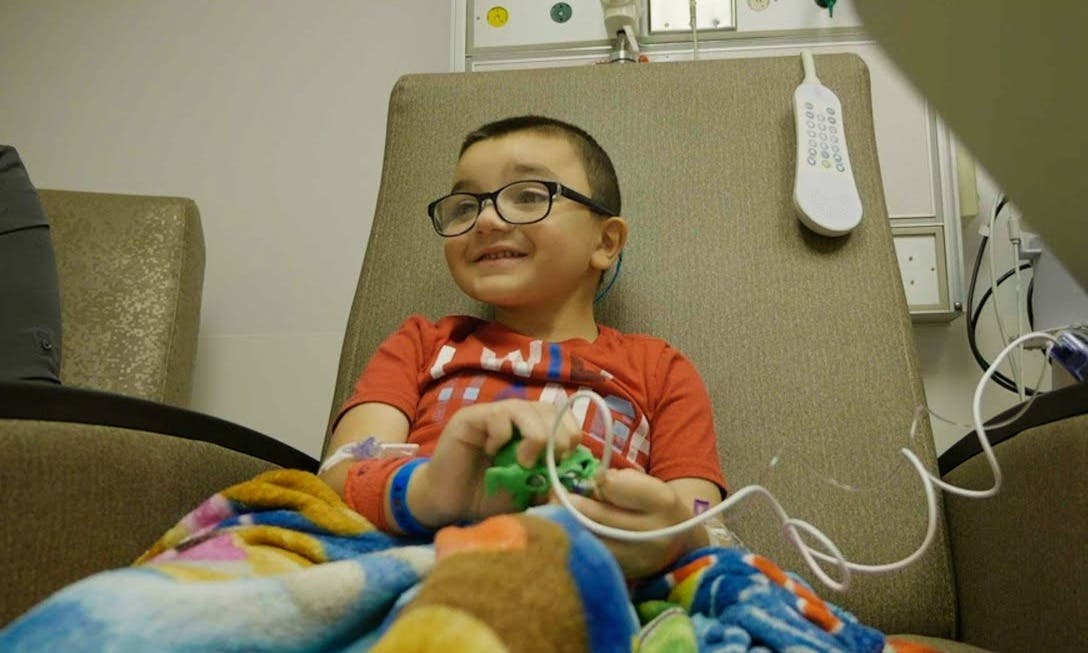
Gideon Griffiths, a 5-year-old from Bellefontaine, Ohio, became the first recipient of this transformative treatment at Nationwide Children’s Hospital. Diagnosed with DMD at birth, Gideon's treatment has become a beacon of hope for countless families across the nation. His journey epitomizes the exhaustive wait for an effective treatment, making his story all the more poignant.
Related Stories
“This is a meaningful day not only for these first families, but for families battling DMD everywhere,” remarked Jerry Mendell, MD, the principal investigator in the Center for Gene Therapy at the Abigail Wexner Research Institute. As a co-inventor of ELEVIDYS, Dr. Mendell added, “It is thrilling to arrive at this moment of getting treatment to a patient population that has waited so long for more hope.”
The gene therapy, named ELEVIDYS (SRP-9001), represents a collaborative effort. Dr. Mendell and Louise Rodino-Klapac, PhD, spearheaded the development. While Dr. Rodino-Klapac was previously associated with Nationwide Children’s, she has since taken on the role of executive vice president, head of Research and Development, and chief scientific officer at Sarepta Therapeutics. This trailblazing gene therapy initiative was licensed by Sarepta in 2018.
The intricacies of ELEVIDYS are truly groundbreaking. The therapy employs an intravenous infusion technique, utilizing a micro-dystrophin gene packaged into an adeno-associated virus serotype, AAVrh74. This specific serotype was isolated at Nationwide Children’s, further showcasing the institution's contributions to the advancement of gene therapy. The primary goal of the treatment is to deliver missing or corrected genes to the affected cells, mitigating the devastating symptoms of DMD.
ELEVIDYS’s journey from conception to FDA approval is testament to the relentless pursuits at Nationwide Children’s Hospital. It's notable that this is not their first foray into gene therapy. In fact, Nationwide Children’s has been at the vanguard of pioneering gene therapies, with two of the only eight gene therapies approved by the FDA to their name.
Erin Griffiths, Gideon's mother, expressed her profound gratitude and hope for the future, saying, “It’s lifechanging to be able to experience this, to be able to give Gideon a better quality of life. We feel hopeful and thankful, and we’re excited to watch our little boy run around, play and just be a boy.”
Elevidys, a Gene Therapy for Duchenne Muscular Dystrophy. (CREDIT: Sarepta)
The accelerated approval of ELEVIDYS by the U.S. Food and Drug Administration (FDA) in June has paved the way for many more children across the country to benefit from this revolutionary treatment. It’s a momentous period for the medical community, and for families with members affected by DMD. The quest for effective treatments has spanned decades, and with the introduction of therapies like ELEVIDYS, the future is looking increasingly hopeful.
This development underscores the importance of persistent research and innovation in the field of neuromuscular medicine. The efforts at the Abigail Wexner Research Institute at Nationwide Children’s Hospital are not only pushing the boundaries of what's possible in medical science but are also offering tangible hope and improved quality of life to those affected by debilitating disorders.
Note: Materials provided above by UBC Faculty of Medicine. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.



