Battery-sized heart pump is lifesaving for kids awaiting heart transplants
The Jarvik 15mm is the latest pump iteration designed specifically for pediatric patients and designed to support these small patients
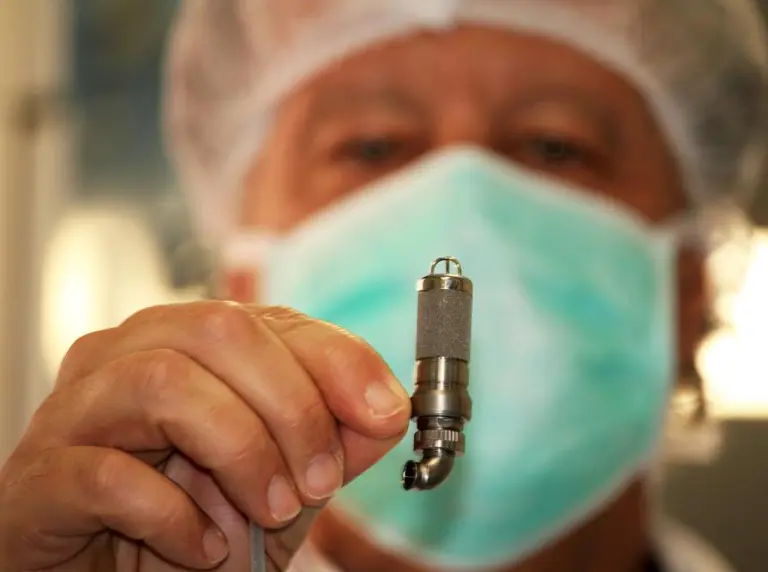
In a groundbreaking advance in pediatric heart care, researchers at Stanford School of Medicine have developed a new, AA battery-sized, implantable device designed to assist children with severe heart failure.
This device, the Jarvik 2015 ventricular assist device (VAD), is compact enough to be implanted in children as light as 18 pounds and has shown promising results in early testing. Unlike the larger, non-implantable Berlin Heart which restricts patient mobility and necessitates prolonged hospital stays, the Jarvik 2015 allows children greater freedom and the potential to wait for a heart transplant at home.
The results of a feasibility trial involving seven children were published in the Journal of Heart and Lung Transplantation. In this trial, six children successfully received heart transplants, and one child's heart function improved to the point where a transplant was no longer necessary.
"This study marks a pivotal step in pediatric heart care, potentially shifting how we support young patients awaiting heart transplants," said Dr. Christopher Almond, the study’s lead author and a pediatric cardiologist at Stanford Medicine.
Dr. Almond explained the significance of this development, noting that pediatric heart care technology has lagged behind adult treatments for decades. "Ventricular assist devices for adults have improved every decade, yet in pediatrics, we’re using technology from the 1960s," he said. The adult versions of these devices are typically implanted inside the chest, allowing patients to lead relatively normal lives during their wait for a transplant.
The need for such a device is critical given the rarity of heart failure in children, which diminishes market incentives for the development of pediatric medical devices. The larger Berlin Heart device, currently the only other option for young children, is cumbersome and linked to a higher risk of stroke.
Children using the Berlin Heart are usually confined to the hospital due to the device's size and the care they require, which not only stresses the patients and their families but also imposes significant costs on the healthcare system and occupies hospital beds that could be used for other critical care needs.
Related Stories
The Jarvik 2015 aims to address these challenges by being significantly smaller—just a bit larger than an AA battery. It is surgically attached to the left ventricle, the heart's main pumping chamber, to help maintain blood flow.
The study’s senior author, Dr. William Mahle, chief of cardiology at Children’s Healthcare of Atlanta, highlighted the device's potential impact: "If the early results are confirmed in larger trials, this could significantly ease the emotional and physical toll on children and their families, as well as reduce hospital stays."
During the trial, the children ranged from 8 months to 7 years old and weighed between 18 to 46 pounds. The device supported them for a median of 149 days as they awaited transplantation or recovery. Despite being part of a clinical trial which required hospitalization, the goal is for future patients to be able to use the device at home.
However, the trial was not without challenges. Some children experienced complications such as strokes or the need for additional support from the Berlin Heart.
Yet, these were within the expected range of complications for such severe cases. Quality of life assessments indicated that the children tolerated the Jarvik 2015 well, with reports of maintained mobility and minimal discomfort.
Encouraged by these initial findings, the National Institutes of Health has funded an expanded trial to further evaluate the device’s effectiveness and safety. This upcoming trial will enroll 22 participants across 14 U.S. medical centers and two European sites.
"Launching the next phase of research is thrilling, and it brings us closer to providing a better quality of life for children with end-stage heart failure," said Dr. Almond.
As researchers continue to develop and refine technologies like the Jarvik 2015, the hope is that these young patients will soon have a safer and more comfortable path while awaiting life-saving heart transplants.
This advancement not only represents a significant leap in pediatric healthcare technology but also illuminates the broader issue of medical device development for rare pediatric conditions, promising a future where children receive as much technological attention as adults.
For more science and technology stories check out our New Innovations section at The Brighter Side of News.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.