50 types of cancer can be detected from a single blood test, study finds
Scientists are inching closer to the realization of a multi-cancer early detection test capable of identifying over 50 forms of cancer.

[July 3, 2023: Staff Writer, The Brighter Side of News]
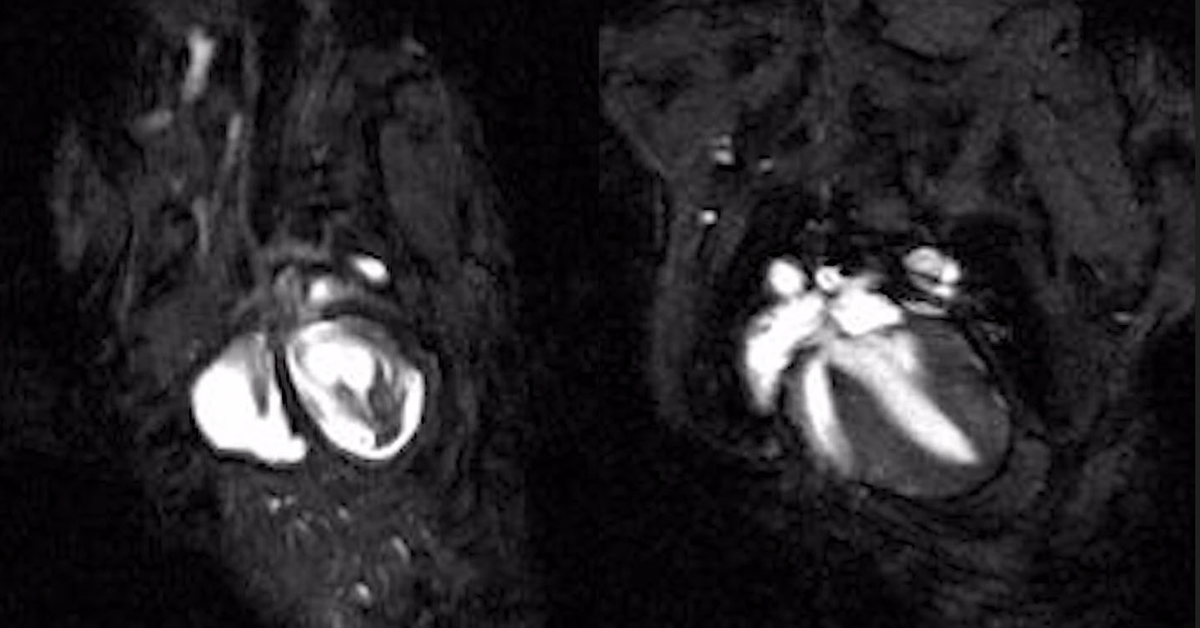
Study findings confirm that the test is proficient in detecting and classifying cell-free DNA (cfDNA). (CREDIT: Creative Commons)
Scientists are inching closer to the realization of a multi-cancer early detection (MCED) test capable of identifying over 50 forms of cancer. This diagnostic tool will be initially accessible to a select group: those aged 50 and above, who are symptom-free yet categorized as high risk for cancer.
Results from the final phase of the Circulating Cell-free Genome Atlas (CCGA) study, recently documented in the Annals of Oncology, support the efficacy of this test.
The research confirms the test's capability to identify and classify cell-free DNA (cfDNA) – remnants from tumors present in an individual's bloodstream with cancer. Impressively, the test can also pinpoint the original tumor's location, even in patients who show no signs related to cancer.
A new cancer screening paradigm
Dr. Eric A. Klein, lead author of the research and Chairman Emeritus of the Glickman Urological & Kidney Institute, thinks that this study aligns with prior findings from a CCGA sub-study, but on a grander scale and with separate verification. He believes these results are paving the way for a fresh perspective on cancer screening.
“With the multi-cancer early detection tests, we have the opportunity to diagnose and treat cancer earlier. Used alongside other screening modalities, this could significantly reduce cancer-related deaths,” he says. For some high-mortality cancers – including liver, pancreatic and esophageal – this is the first screening test available.
Related Stories:
Presently, patients in the United States have access to a mere five screening tests for different types of cancer, namely prostate, lung, breast, colorectal, and cervical cancers. Each of these tests bears its own set of constraints, from degrees of invasiveness to inconsistencies in application across various clinical settings, as well as a high rate of false positives that can result in overdiagnosis and unnecessary treatments.
The advent of this innovative assay ushers in the potential for a transformative shift in cancer screening. Its key ability is the detection of circulating cfDNA from a single blood sample, proving especially useful in identifying more aggressive and advanced-stage cancers, which are thought to produce greater quantities of cfDNA.
Yet, this also emphasizes the necessity to integrate this new technique, the MCED, with the current screening methodologies until further advancements are achieved. “Prostate cancer, for example, sheds comparatively less DNA than other tumors, making it less likely to be detected by the novel assay,” explains Dr. Klein, a urologic oncologist.
MCED test performance for cancer signal detection (A) overall sensitivity by cancer class. (CREDIT: Annals of Oncology)
How does it work? A genomic sequencing technology elucidates methylation, or chemical changes in the DNA that control gene expression, coupled with a machine learning application that systematically identifies patterns of irregularities in the DNA indicative of cancer. These patterns provide evidence as to where the cancer orginated and can help guide further diagnostic testing.
GRAIL, Inc. a California-based biotech company, developed the assay and has funded international research efforts. The MCED test is now available in the United States by prescription only.
Key findings
The study evaluated the performance of the test in two cohorts: individuals already diagnosed with cancer (n = 2,823) and those without a cancer diagnosis (n = 1,254). It detected cancer signals from more than 50 types of cancer across all four stages of disease.
The test’s overall sensitivity across cancer types and stages was 51.5%; sensitivity increased with each stage – the more advanced the disease, the more sensitive the test.
The average rate of sensitivity in cancers stages I – III was 67.6% in 12 pre-specified cancers (anal, bladder, bowel, esophageal, stomach, head and neck, liver and bile-duct, lung, lymphoma, ovarian, pancreatic, and cancers associated with white blood cells), which account for almost two-thirds of cancer-related deaths in the US.
The test’s specificity (also known as false-positive rate) was 99.5%, meaning that it found a false signal for cancer in only 0.5% of those tested.
In 88.7% of cases, the test correctly identifies the tissue in which the cancer was located, which could help decrease the time to diagnosis and allow physicians to facilitate treatment with greater efficiency.
A population-based screening tool?
Dr. Klein says the team is satisfied with the promising findings; they are hopeful that this technology could be extrapolated as a tool for cancer screening at a population level.
In fall 2020, Cleveland Clinic announced that it would begin enrollment for its arm of the PATHFINDER study, of which Dr. Klein is the principal investigator. He explains that the strength of the CCGA study is its robust assessment of the assay itself. The PATHFINDER study, however, is intended to evaluate the care pathways from a cancer “signal detected” test in a primary care setting to arriving at a diagnostic resolution with a cancer specialist.
Accuracy of CSO prediction (confusion matrix). The top panel indicates overall accuracy of CSO prediction. The bottom panel depicts a confusion matrix showing accuracy (top horizontal axis) and precision of CSO prediction by CSO (right vertical axis) among true positive participants with a known cancer signal origin. (CREDIT: Annals of Oncology)
“We can say with confidence that the multi-cancer early detection test has clinical utility. We still don’t know the implications for its use in a more generalizable patient population, but the results are very promising.”
The 50+ types of cancers tested:
1. Adrenal Cortical Carcinoma
2. Ampulla of Vater
3. Anus
4. Appendix, Carcinoma
5. Bile Ducts, Distal
6. Bile Ducts, Intrahepatic
7. Bile Ducts, Perihilar
8. Bladder, Urinary
9. Bone
10. Breast
11. Cervix
12. Colon and Rectum
13. Esophagus and Esophagogastric Junction
14. Gallbladder
15. Gastrointestinal Stromal Tumor
16. Gestational Trophoblastic Neoplasms
17. Kidney
18. Larynx
19. Leukemia
20. Liver
21. Lung
22. Lymphoma (Hodgkin and Non-Hodgkin)
23. Melanoma of the Skin
24. Mesothelioma, Malignant Pleural
25. Merkel Cell Carcinoma
26. Nasal Cavity and Paranasal Sinuses
The 50+ types of cancers tested (cont.):
27. Nasopharynx
28. Neuroendocrine Tumors of the Appendix
29. Neuroendocrine Tumors of the Colon and Rectum
30. Neuroendocrine Tumors of the Pancreas
31. Oral Cavity
32. Oropharynx (HPV-Mediated, p16+)
33. Oropharynx (p16-) and Hypopharynx
34. Ovary, Fallopian Tube and Primary Peritoneum
35. Pancreas, exocrine
36. Penis
37. Plasma Cell Myeloma and Plasma Cell Disorders
38. Prostate
39. Small Intestine
40. Soft Tissue Sarcoma of the Abdomen and Thoracic Visceral Organs
41. Soft Tissue Sarcoma of the Head and Neck
42. Soft Tissue Sarcoma of the Retroperitoneum
43. Soft Tissue Sarcoma of the Trunk and Extremities
44. Soft Tissue Sarcoma Unusual Histologies and Sites
45. Stomach
46. Testis
47. Uterus, Carcinoma and Carcinosarcoma
48. Uterus, Sarcoma
49. Ureter (and Renal Pelvis)
50. Vagina
51. Vulva
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.