Breakthrough new drug could prevent or treat Alzheimer’s disease, study finds
Researchers at Gladstone Institutes make a groundbreaking discovery about the APOE4 gene variant and Alzheimer’s disease.
[Oct. 22, 2023: Staff Writer, The Brighter Side of News]
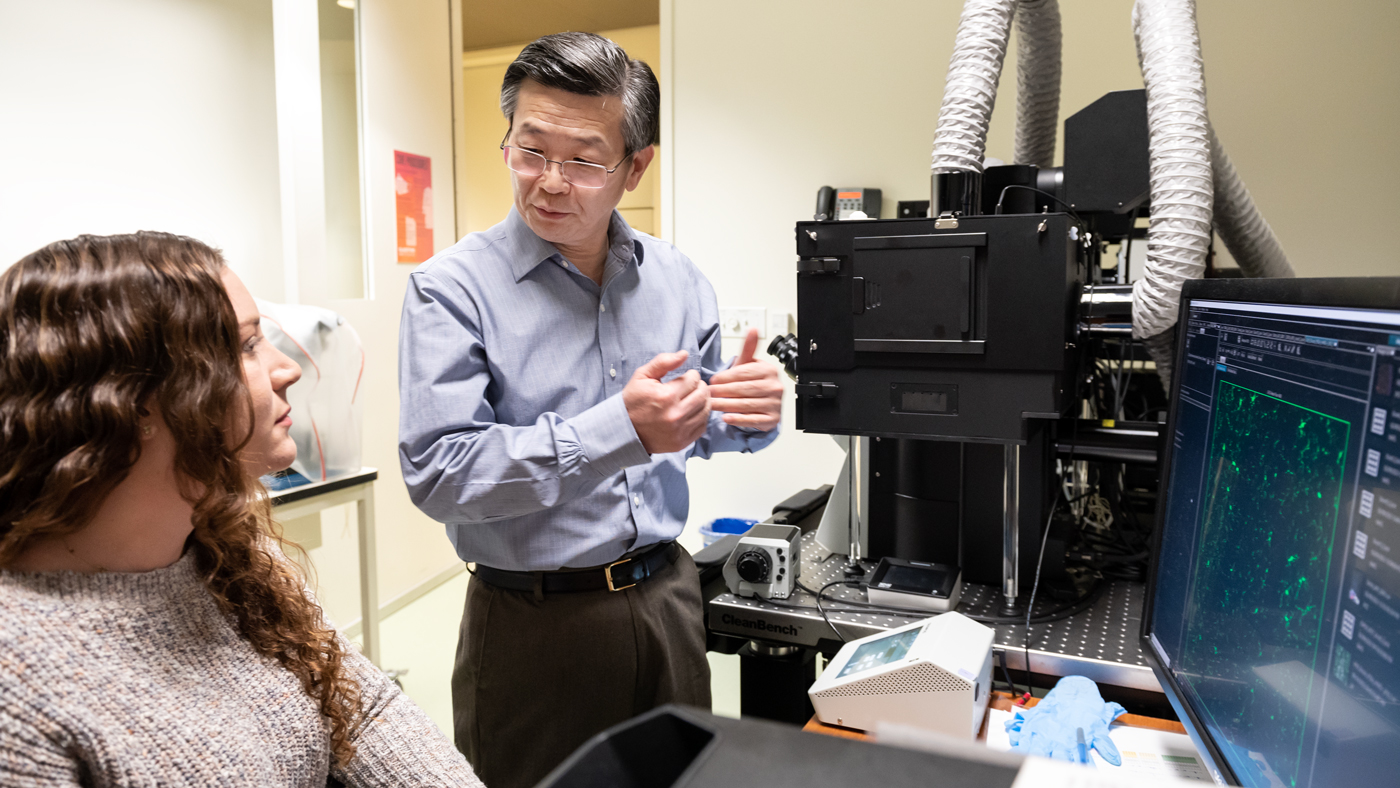
Huang (right) and his team, including Koutsodendris (left), created a mouse model for Alzheimer’s disease to study specifically the production of ApoE4 by neurons. (CREDIT: Gladstone Institutes)
In an era where personalized medicine is becoming a reality, understanding the genetic factors behind Alzheimer’s disease is crucial. Particularly, the APOE4 gene variant, present in roughly one-quarter of the population, has been a point of focus due to its significant association with an increased risk of developing Alzheimer's.
However, the precise mechanism by which APOE4 influences this risk has largely remained an enigma. Today, a revelatory study from the scientists at Gladstone Institutes is changing this narrative, shedding light on the potential pathway through which APOE4 exacerbates neurodegenerative processes.
The research, recently published in the esteemed journal Nature Aging, illuminates how neurons harboring the APOE4 variant exhibit a proclivity for releasing a specific immune signaling molecule, known as High Mobility Group Box 1 (HMGB1).
This molecule, under normal circumstances, plays a pivotal role in several cellular processes. However, when overexpressed, HMGB1 initiates a domino effect, activating brain immune cells termed microglia, subsequently igniting inflammation and contributing to the degeneration of neurons, hallmark features of Alzheimer's disease.
Related Stories
Dr. Yadong Huang, MD, PhD, a leading investigator at Gladstone and a professor of neurology and pathology at the University of California, San Francisco, expressed his team's surprise at the findings.
“We were quite surprised and excited that targeting this pathway leads to such strong protection against APOE4-driven neurodegeneration,” Huang commented, emphasizing the results' potential implications for understanding and eventually treating Alzheimer's disease.
Unraveling the Alzheimer’s Conundrum: What Triggers What?
Alzheimer's disease orchestrates a symphony of destruction within the brain, with diverse effects on various cell types. The pathological hallmarks include the buildup of proteins such as tau and amyloid-beta, microglial activation leading to inflammation, and the subsequent degeneration and death of neurons. Yet, a pressing question that has perplexed scientists for years is what comes first in this catastrophic cascade.
Yadong Huang and his team demonstrated in mice that the ApoE4 protein from neurons plays a much bigger disease-driving role in Alzheimer’s than previously thought. (CREDIT: Gladstone Institutes)
Huang reflects on this dilemma, “It could be that neurons degenerate first, and that triggers the activation of microglia. But it could also be that microglia become activated first and then trigger the neurodegeneration.” This classic chicken-or-egg scenario underscores the complexity of Alzheimer's disease and the intricate interplay of its pathological elements.
To dissect this conundrum, Huang’s team zeroed in on microglial activation within the context of APOE4, a gene variant known to be a substantial genetic risk factor for Alzheimer's.
The APOE gene exists in three variants: APOE2, APOE3, and APOE4. APOE3 is the most common, while APOE4 carries notorious connotations for brain health. Individuals with one copy of APOE4 face a 3.5-fold increase in Alzheimer's risk compared to those with APOE3, while those with two copies of APOE4 confront a staggering 12-fold risk escalation.
In their investigative pursuit, Huang, alongside colleagues including Dr. Nicole Koutsodendris, a PhD and former graduate student in Huang’s lab, questioned whether APOE4-variant neurons produced signaling molecules capable of rousing microglia. Their attention quickly converged on HMGB1, known for its role in triggering potent inflammatory responses in certain cancers and infections.
The team embarked on initial experiments examining the location of HMGB1 in neurons within the brains of Alzheimer's mouse models carrying either the APOE3 or APOE4 variants. What they found was nothing short of striking.
“We saw really striking patterns,” recounts Koutsodendris. “When neurons with APOE4 were exposed to stress, HMGB1 moved from the nucleus to the cytoplasm of the cells and was then released into the space outside the cell, whereas in neurons with APOE3, HMGB1 remained inside the nucleus.”
These observations were bolstered by subsequent studies in mouse models, demonstrating that the selective removal of APOE4 from neurons halted HMGB1's release, thereby reinforcing the connection between neuronal APOE4 and HMGB1 discharge.
Tau pathology and its spread are significantly reduced after neuronal APOE4 removal. (CREDIT: Nature Aging)
Identifying a New Therapeutic Target
In a conclusive series of experiments, the research team explored the effects of two experimental drugs known to impede HMGB1 release. Their findings were groundbreaking; the drug combination curtailed microglial activation and the progression of neurodegeneration in dementia-related mouse models expressing APOE4.
“It was a very clear result: when you block the exit of HMGB1 out of neurons, you protect these mice from many different aspects of Alzheimer’s disease pathology,” Huang asserts, underscoring the potential of this therapeutic strategy.
While APOE4's influence on neurons is multifaceted, the researchers propose that in the absence of microglial activation, other effects of APOE4 may not sufficiently drive neurodegeneration. This insight has steered Huang and his team toward a conviction that medications targeting HMGB1 could be instrumental in prophylaxis or treatment strategies for APOE4-associated Alzheimer's disease.
Koutsodendris (right), a former trainee in Huang’s (left) lab, helped show that removing ApoE4 produced by neurons—even though it only accounts for a small portion of this protein in the brain—leads to significant reductions in the pathology of Alzheimer’s disease. (CREDIT: Gladstone Institutes)
Promisingly, Huang notes, “These small molecules have already been in clinical trials for other diseases, and so they could potentially move quickly into trials for Alzheimer’s disease.” Nevertheless, the team is far from complacent, continuing their exploration of alternative methods, including new drug candidates, to target HMGB1, its neuronal release, and its subsequent effects on microglia.
This groundbreaking study not only demystifies the role of APOE4 in Alzheimer's but also ignites hope for those carrying the gene variant. With the potential fast-tracking of drugs into clinical trials, the future of Alzheimer's treatment appears increasingly optimistic. The dedication of researchers like Huang and his team at Gladstone Institutes is a testament to the relentless pursuit of knowledge and healing, illuminating a path forward in the battle against this formidable disease.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.



