Breakthrough research transforms aggressive cancer cells into healthy muscle
Researchers have found a way to morph rhabdomyosarcoma cells into muscle cells. As the cells transform, they take on typical muscle features
[Aug. 29, 2023: Staff Writer, The Brighter Side of News]
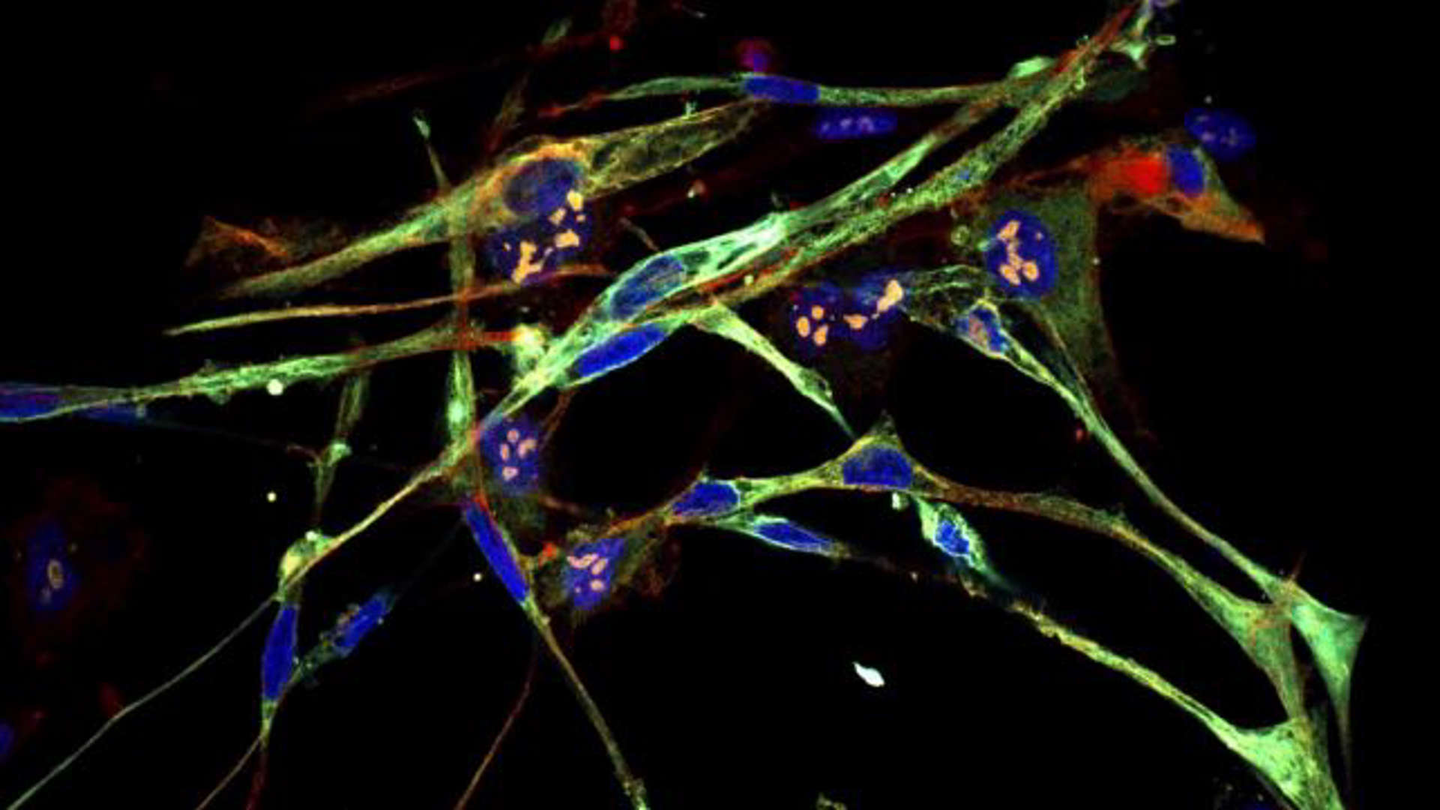
Cold Spring Harbor Laboratory Professor Christopher Vakoc and CSHL School of Biological Sciences graduate Martyna Sroka have found a way to morph rhabdomyosarcoma cells into muscle cells. As the cells transform, they take on typical muscle features, including the spindle-like shape seen here. (CREDIT: Vakoc lab/Cold Spring Harbor Laboratory)
A new dawn may be breaking in the battle against one of the most aggressive forms of childhood cancer, rhabdomyosarcoma, which originates in muscle tissue. Scientists have accomplished an extraordinary feat by prompting these cancer cells to metamorphose into healthy muscle cells.
This could be a monumental step towards developing novel therapies against the disease and possibly offer insights for the treatment of other human cancers.
The Cellular Transformation
Molecular biologist Christopher Vakoc, from the renowned Cold Spring Harbor Laboratory, describes this cellular conversion with palpable enthusiasm. "The cells literally turn into muscle," he explains. "The tumor sheds all characteristics of cancer. It’s akin to switching gears from a cell that's perpetually in replication mode to cells that are singularly focused on contraction. All their resources are channeled into contraction, effectively inhibiting their capacity to revert to a proliferative state."
However, the journey of understanding cancer isn't straightforward. Unlike the image of a singular monstrous entity, cancer varies depending on the cell it originates from.
Related Stories
Rhabdomyosarcoma is notably aggressive, primarily afflicting children and teenagers. Its onset occurs in the skeletal muscles when cells mutate, multiplying uncontrollably, and eventually overwhelming the body. The statistics paint a grim picture with survival rates for those with an intermediate risk ranging between 50 to 70 percent.
Enter differentiation therapy, a beacon of hope in the otherwise dark landscape of cancer treatment. This therapy took root from an intriguing observation: leukemia cells closely resemble undifferentiated stem cells, which are yet to mature into distinct cell types. Differentiation therapy essentially propels these cells down the path of maturation, urging them to evolve into specific mature cell types.
Vakoc and his research squad had previously pulled off a remarkable achievement. They had reversed the cancerous mutation in cells associated with Ewing sarcoma, another vicious childhood cancer that predominantly appears in bones. Their ambitious goal was to replicate this success with rhabdomyosarcoma, despite predictions that differentiation therapy for this type of cancer was possibly decades away.
This picture shows the typical microscopic appearance of rhabdomyosarcoma. (CREDIT: Creative Commons)
Unlocking the Genetic Puzzle
In pursuit of this aim, the research team employed an advanced genetic screening methodology. They sought to identify genes capable of propelling rhabdomyosarcoma cells into full-fledged muscle cells. Their answer was enshrined in a protein named Nuclear transcription factor Y (NF-Y).
A specific protein, PAX3-FOXO1, is generated by rhabdomyosarcoma cells, and this protein is pivotal for the cancer's spread. Essentially, the cancer leans heavily on this protein. Astonishingly, the researchers pinpointed that deactivating NF-Y neutralizes PAX3-FOXO1. This cessation nudges the cells to advance in their developmental journey, culminating in their transformation into mature muscle cells devoid of any cancerous traces.
The cartoon model above illustrates RMS cells’ transformation to healthy muscle cells. When NF-Y is depleted from the cells, the cancer stops multiplying and starts to take on typical muscle features and functions. The microscopy images on the bottom row capture real cells before and after this transformation. (CREDIT: Vakoc lab/Cold Spring Harbor Laboratory)
The implications of this discovery are profound. This vital step could drastically hasten the development of differentiation therapy for rhabdomyosarcoma.
Furthermore, having tested and proved their technique on two distinct types of sarcoma, the research team posits that their approach could be adapted for other forms of sarcoma and potentially various cancer types. Their methodology arms scientists with the requisite tools to decipher how to trigger differentiation in cancer cells.
Recurrent chromosomal rearrangements found in rhabdomyosarcoma (RMS) produce the PAX3–FOXO1 fusion protein, which is an oncogenic driver and a dependency in this disease. (CREDIT: Vakoc lab/Cold Spring Harbor Laboratory)
As Vakoc poetically puts it, "Every successful medicine has its origin story. Research undertakings like this are the fertile grounds from which groundbreaking drugs emerge."
The findings of this groundbreaking research are now accessible in the esteemed 'Proceedings of the National Academy of Sciences'.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.



