E. coli based ‘smart microbe’ is a game changer in treating IBD and other gut diseases
Researchers have succeeded in developing an E. coli-based “smart microbe” that secretes therapeutic payloads and antibodies, into the gut.
[Apr. 3, 2023: JJ Shavit, The Brighter Side of News]
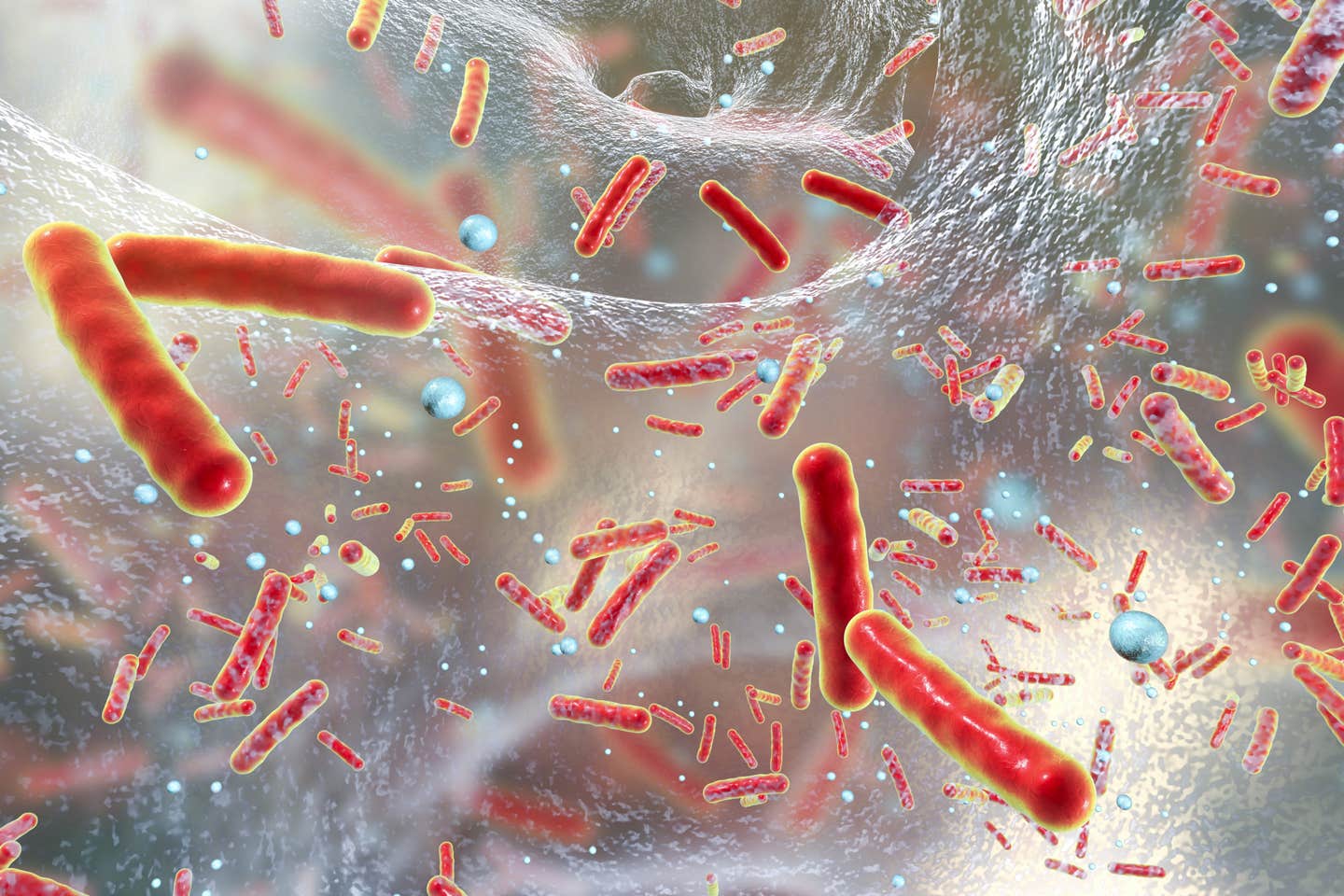
The smart microbe was as efficacious as a systemically delivered conventional monoclonal antibody in limiting colitis in inflammatory bowel disease. (CREDIT: Creative Commons)
Researchers at Massachusetts General Hospital (MGH) are pioneering a new field of genetic modification by engineering probiotic bacteria to secrete therapeutic proteins at sites of disease. The technique, which has shown efficacy in mice, aims to improve the delivery of drugs by bypassing the systemic administration of conventional drugs that often have serious side effects.
The research, published in the latest issue of Cell Host & Microbe, suggests that genetically modifying probiotic E. coli could be used to treat a range of human diseases, including inflammatory bowel disease (IBD), Clostridiodes difficile (C. diff) infections, and even solid tumors.
Humans are colonized with thousands of bacterial strains, including the well-known gut bacteria E. coli. The bacteria can be genetically modified to secrete therapeutic payloads at the site of disease, enhancing their intrinsic therapeutic properties. In this case, the MGH researchers focused on engineering E. coli to secrete proteins of therapeutic value into its surroundings.
The team modified E. coli to secrete an antibody that blocks inflammation, which proved as efficacious as a systemically delivered antibody in limiting the development of colitis in a mouse model of IBD.
Related Stories:
One of the challenges of enhancing the therapeutic capabilities of beneficial microbes like E. coli was to enable them to secrete proteins into their surroundings. E. coli are surrounded by an outer envelope across which few proteins are transported.
Senior author Cammie F. Lesser, MD, PhD, and her team at MGH have been studying complex protein secretion systems for more than two decades with the goal of reengineering them as drug delivery systems. Using knowledge gained from fundamental-based research, the lab introduced a version of this secretion machine into E. coli and modified it to secrete proteins into its surroundings.
The resulting programmable platform is referred to as PROT3EcT for probiotic type III secretion E. coli. As proof of the potential therapeutic value of PROT3EcT, Lesser and her colleagues tested the engineered E. coli in a mouse model of inflammatory bowel disease. The E. coli engineered to secrete nanobodies that bind to and inhibit tumor necrosis factor (TNF) alpha, a pro-inflammatory cytokine, was as effective in blocking the development of inflammation in the intestines of mice as an injected monoclonal antibody that targets the same cytokine.
Graphical abstract: PROT3EcT secrete functional single-domain antibodies, nanobodies (Nbs), and stably colonize and maintain an active secretion system within the intestines of mice. (CREDIT: Cell Host & Microbe)
Monoclonal antibodies that neutralize TNF alpha result in general suppression of the immune system, which can have unintended effects. By using engineered bacteria, it may be possible to deliver these anti-inflammatory antibodies and limit immunosuppression directly to where inflammation is present. Lesser and her colleagues are now working on engineering bacterial strains that will secrete therapeutic proteins in response to specific conditions, such as when inflammation begins developing in the gut.
Engineered E. coli can also be outfitted to secrete antibodies that block toxins released by harmful strains of bacteria. Lesser’s team is investigating the microbe’s potential to treat intestinal infections such as Clostridiodes difficile (C. diff), colitis, and other toxin-driven infections. E. coli and other bacteria also replicate in solid tumors, so Lesser and others are researching the use of engineered microbes as anti-cancer agents.
Engineering of PROT3EcT, E. coli outfitted with a programmable protein secretion system. (CREDIT: Cell Host & Microbe)
“We hope to advance these strains towards the treatment of a variety of human diseases by outfitting them to secrete a variety of proteins of therapeutic value,” says Lesser. While the technology is still in its early stages and many challenges remain, including regulatory approval, the potential for genetically modifying probiotic bacteria to treat a wide range of human diseases is exciting.
Lesser’s research could lead to the development of smart microbes that release therapeutic payloads at sites of disease, thus maintaining therapeutic efficacy while limiting the side effects that can be associated with the systemic administration of conventional drugs. This technology could revolutionize the way we treat a range of diseases, from inflammatory bowel disease to cancer.
Pretreating with anti-TNF-α-secreting PROT3EcT limits colitis in a preclinical IBD model. (CREDIT: Cell Host & Microbe)
The potential of genetically modifying bacteria for therapeutic purposes has been recognized for several decades. However, the technology has only recently become more accessible and affordable due to advances in genetic engineering and synthetic biology.
In addition to the work being done at MGH, other researchers are also exploring the use of engineered bacteria for therapeutic purposes. For example, scientists at the University of California, San Diego, are working on developing bacteria that can produce cancer-killing toxins in response to specific signals in tumors.
Another research team at the University of Texas at Austin is engineering bacteria to produce drugs that could treat diseases such as hypertension and high cholesterol.
Despite the potential benefits of this technology, there are also concerns about the safety and ethics of genetically modifying bacteria. Critics argue that the release of genetically modified bacteria into the environment could have unintended consequences and potentially harm ecosystems.
To address these concerns, researchers working with genetically modified bacteria are taking measures to minimize the risk of unintended release. For example, the bacteria used for therapeutic purposes are typically engineered to be unable to survive outside of a laboratory setting.
Additionally, researchers are working with regulatory agencies to ensure that genetically modified bacteria are developed and used safely and responsibly.
Despite these challenges, the potential benefits of genetically modifying bacteria for therapeutic purposes are significant. As the technology continues to advance, it could provide new and innovative ways to treat a range of diseases, while minimizing the side effects associated with conventional drugs.
For patients with chronic conditions such as inflammatory bowel disease, the development of smart microbes that release therapeutic payloads at sites of disease could offer new hope for effective treatment with fewer side effects.
While there is still much work to be done before this technology becomes widely available, the progress being made by researchers such as Cammie Lesser and her colleagues at MGH is a promising step towards a new era of personalized, targeted therapies.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.