Gene-edited fungus tastes like meat and cuts protein’s climate impact by more than 50%
A new CRISPR-edited fungus offers a cleaner and more efficient way to produce protein, with far less impact on the environment.

 Edited By: Joseph Shavit
Edited By: Joseph Shavit
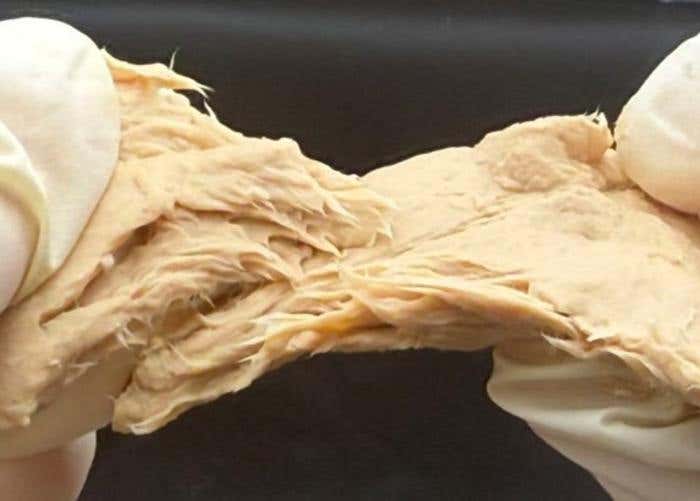
A picture of Fusarium venenatum. Gene-edited mycoprotein cuts emissions, land use, and resource demand while improving nutrition. (CREDIT: Xiao Liu)
The way the world grows protein is straining the planet. Raising animals for food covers close to 40 percent of all farmland, and it releases nearly 14.5 percent of global greenhouse gases. As populations grow and nations worry about long-term access to food, this system looks harder to sustain. Scientists are searching for options that protect the climate while still giving you nutritious food.
One promising direction comes from fungi. A meat-like protein called mycoprotein has gained attention because some strains grow fast, taste familiar, and use far fewer resources than livestock. Yet even this approach has limits. These fungi grow inside giant metal tanks filled with sugar-rich feed, and many strains have thick cell walls that lock away valuable nutrients. Earlier studies showed good environmental results, but they rarely tested what happens when you scale production to the size needed to feed millions of people.
A team from Jiangnan University decided to look at both challenges at once by improving the nutrition of a well-known fungus while cutting the resources needed to grow it.
How Gene Editing Changed a Familiar Fungus
The fungus Fusarium venenatum already appears in foods sold in the United States, the European Union, and many other places. Its natural fibers give it a texture similar to meat. Still, the cell walls can be tough to digest because they contain chitin and several types of glucans. That structure helps the fungus survive in nature, but it slows the release of protein during digestion.
The researchers turned to CRISPR gene editing, which let them remove two genes without adding any outside DNA. One gene controlled chitin synthase, which helps build the cell wall. The other made pyruvate decarboxylase, an enzyme tied to the fungus’s energy use. When both genes were removed, the new strain, named FCPD, formed thinner cell walls and used sugar more effectively.
Before heating, FCPD carried 29 percent less chitin than the original strain. After heating, used to lower nucleic acid levels for food safety, the proportion of chitin rose again because some material leaked out. The pattern was similar for protein content. Even with these changes, the new strain offered a better essential amino acid score and index, two important measures of protein quality. Its digestibility dropped slightly because the mycelium structure shifted, but the overall nutritional profile still improved.
Microscopy showed how different the two strains looked after staining. Wild type mycelia had bright, thick rings around their cell walls, while the edited strain had a more open structure. These changes helped shape how the fungus behaved in fermentation tanks.
Inside Industrial-Scale Production
When grown at industrial scale, both strains formed tangled, thread-like clusters. Even so, FCPD clearly performed better. It needed about 44 percent less glucose to produce the same amount of biomass. Its glucose-to-protein conversion rate more than doubled, and overall production speed rose by 88.4 percent.
To understand why, the team studied which genes and metabolites changed. They found more than a thousand genes expressed differently in the edited strain. Many belonged to pathways tied to carbon and nitrogen use. The glycolysis and gluconeogenesis pathway, which handles glucose breakdown, was downregulated. This helped explain the slower glucose consumption. Other metabolic shifts suggested that the fungus redirected carbon through the TCA cycle in ways that raised protein production.
Both strains were tested in continuous fermentation at an industrial facility over seven days. Safety checks confirmed that none of the common fungal toxins, including deoxynivalenol and zearalenone, appeared in either strain. Heat treatment reduced nucleic acids to approved levels but also caused major biomass loss. The usable yield after heating fell to about 35 percent. The team noted that the remaining broth held valuable nutrients that could be turned into fertilizer, although this idea was outside the main goals of the study.
Measuring the Environmental Footprint
The researchers built a full life cycle model to test how FCPD would perform if one million kilograms of mycoprotein were produced per year. They ran the model across eight energy scenarios in different countries. The edited fungus reduced environmental impacts by about 4 percent to more than 61 percent compared with the original strain, depending on the category and energy mix.
Glucose was the largest driver of climate impact. It contributed up to 99 percent of land use and up to two thirds of greenhouse gas emissions. Electricity mattered more in countries that rely heavily on coal. In places with cleaner grids, electricity had lower climate impacts but raised ionizing radiation scores because of nuclear power.
When compared with other protein sources, FCPD stacked up well. Pea protein had the lowest footprint, but fungal protein still beat chicken in several categories, including climate impact, land use, and freshwater pollution. Cell-cultured meat performed worst because it needs high amounts of sugar, salts, and electricity.
Looking Ahead to Gene-Edited Foods
The CRISPR edits used here did not introduce foreign DNA, and foods produced this way do not require special labeling in the United States. “There is a popular demand for better and more sustainable protein for food,” says corresponding author Xiao Liu. “We successfully made a fungus not only more nutritious but also more environmentally friendly by tweaking its genes.”
First author Xiaohui Wu adds, “A lot of people thought growing mycoprotein was more sustainable, but no one had really considered how to reduce the environmental impact of the entire production process, especially when compared to other alternative protein products.”
The study also points out that China is developing guidelines for gene-edited microbial ingredients that match this approach. Several gene-edited crops and animals are already approved around the world, although regulations vary by region.
Researchers say that similar edits might improve other fungi used in food. They also ask how production could be refined to meet safety limits without losing as much biomass. These questions will guide future work in the field.
Practical Implications of the Research
This research shows how gene editing can help create protein sources that take less land, energy, and water to produce. If scaled widely, foods made from strains like FCPD could ease pressure on agriculture, cut greenhouse gases, and provide high-quality protein in places where farmland or clean water is limited.
The approach also offers a path toward safer, more nutrient-dense meat alternatives that still meet global demand for familiar textures and flavors.
Research findings are available online in the journal Trends in Biotechnology.
Related Stories
- Chef uses fungi to transform food waste into gourmet delights
- Researchers reveal the bacteria and fungi living in the foods we eat
- Fungi-based meat alternatives to help save Earth’s forests
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Writer and Editor
Joshua Shavit is a NorCal-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a co-founder of The Brighter Side of News, he focuses on positive and transformative advancements in technology, physics, engineering, robotics, and astronomy. Joshua's work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.