MIT researchers build universal cancer-fighting immune cells
Engineered CAR-NK cells from MIT and Harvard evade immune rejection, offering faster, safer, off-the-shelf cancer treatment.
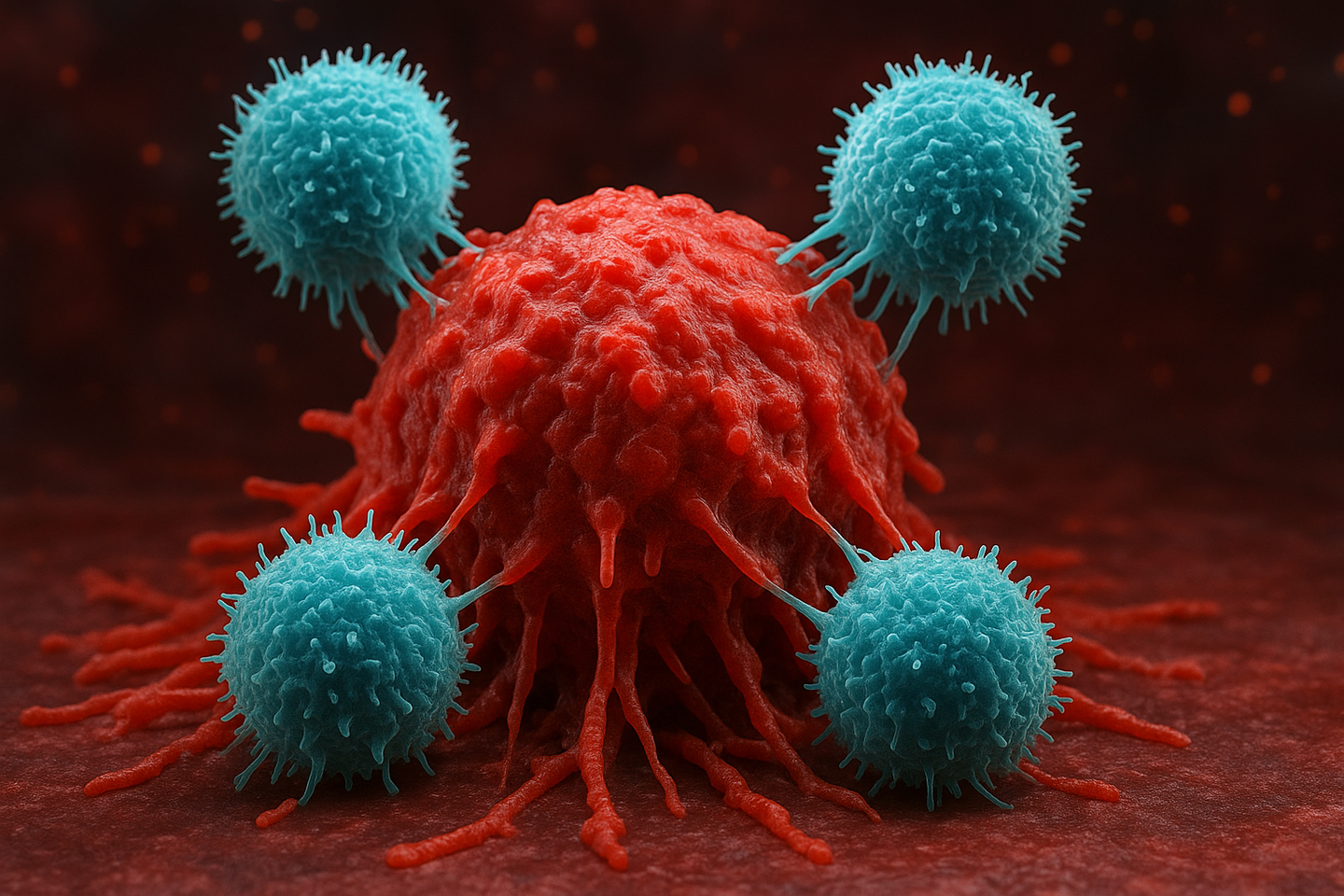
Researchers at MIT and Harvard have engineered stealth CAR-NK cells that avoid immune rejection and attack tumors more effectively. (CREDIT: AI-generated / The Brighter Side of News)
In a breakthrough cancer therapy development, scientists at MIT and Harvard Medical School created a new way of designing immune cells that could make "off-the-shelf" cancer medications more potent and less likely to be rejected by the immune system of patients. The discovery could result in quicker, safer, and more convenient cell-based therapies.
The Trouble with Rejection
Immune cell treatments like CAR-T cells have transformed cancer therapy for a few patients, particularly those with blood malignancies. The treatments reprograms a patient's own T cells to recognize and destroy cancer cells. However, it takes time to make them, is costly, and at times when a patient's immune cells are weakened too much.
That is why researchers have been developing versions that include healthy donor cells that can be pre-prepared and kept in the freezer to be used at a moment's notice. The problem is that when a donor's immune cells are transferred into a patient, the patient's immune system will recognize them as foreign and kill them.
The new research, conducted by MIT biologist Jianzhu Chen and Harvard oncologist Rizwan Romee, aims at a different immune cell: the natural killer, or NK, cell. NK cells already specialize in targeting cancer and virus-infected cells, but they don't cause a lethal complication called graft-versus-host disease that can occur with donor T-cell therapy. In spite of this, donor NK cells can still be destroyed by the patient's immune system before they can function.
The scientists set out to design NK cells that could "hide" from immune rejection without losing their tumor-killing ability.
Engineering Immune Stealth
The scientists used one genetic "construct" that puts three smart changes into donor NK cells at once.
First, they knocked out genes for a group of cell-surface proteins known as HLA-A, B, and C—molecular markers that the immune system uses to differentiate "self" from "foreign." By diminishing these markers but preserving another variant known as HLA-E, the altered cells shielded themselves against the patient's T cells but exposed themselves to other NK cells for attack.
Second, the researchers added an immune-suppressing molecule such as PD-L1 or a synthetic version of HLA-E called SCE to the engineered NK cells. PD-L1 works by binding to the PD-1 receptor on T cells and calming them down. The SCE protein protects the engineered NK cells from being destroyed by the patient's own NK cells.
Finally, they inserted a chimeric antigen receptor—abbreviated to CAR—into the same construct. It is a molecular detector that enables the genetically modified NK cell to recognize and kill cancer cells that bear specific cancer markers, such as CD19 on lymphoma cells.
"All three components—gene silencing, immune evasion, and tumor targeting—are delivered in one step," Chen said. "These cells kill cancer better, for longer durations, and they're safer."
Surviving the Immune Gauntlet
In laboratory tests, the engineered NK cells were far more resistant than natural NK cells. When exposed to aggressive T cells, untreated NK cells were completely wiped out, while HLA-ABC silenced NK cells remained virtually intact. When PD-L1 or SCE was added, they were even more resistant.
In mouse experiments, the result was spectacular. Mice that had human immune systems were treated with donor NK cells with human immune cells and lymphoma tumors. In those who received the unmodified cells, NK cells dropped off in a matter of weeks, and tumors spread unchecked. But in mice who had the stealth CAR-NK cells, the engineered immune cells survived for weeks, attacked the tumors, and essentially killed the cancer.
Even better still, the mice had fewer side effects that cause inflammation. Inflammation biomarkers such as IL-6 and TNF—molecules known to cause life-threatening bouts of the immune system storming—were drastically reduced in mice subjected to conventional CAR therapies.
Toward Ready-Made Cancer Immunotherapy
It currently takes weeks to produce CAR-based therapies for each patient, and in emergency care situations, that holds up treatment. The new one-step method might eliminate that by allowing donor NK cells to be mass-produced, frozen, and available on demand upon diagnosis.
"This enables one-step engineering of CAR-NK cells that are capable of evading rejection by host immune cells," Chen said. "They are more potent in killing cancer cells and they're safer."
Romee, a Dana-Farber Cancer Institute immunotherapy expert on cancer, said the findings suggest donor NK therapies could become "universal"— usable in any patient without immune matching.
While the current study was conducted using blood cancers such as lymphoma, the approach would be scalable to solid tumors by designing CARs targeting proteins unique to such tumors. The same stealth technology could also be applied to treat autoimmune disorders, where the immune system causes destruction of normal tissue.
Chen's group already is collaborating with a local biotech company to attempt to treat lupus, an autoimmune disease, with engineered NK cells. And they are going to extend their cancer therapy to early-stage human trials in collaboration with scientists at Dana-Farber.
What Happens Next
Before these stealth NK cells can be applied to humans, scientists will need to establish that they are safe and act over the long term in animals and clinical trials. Regulators also need to ensure that silencing immune markers does not introduce new risks, such as unwanted infection or immune system confusion.
However, scientists see it as a significant step toward universal immune-cell therapies—those that can be manufactured in advance, stored in hospitals, and administered into patients in days instead of weeks.
If successful, this development could introduce a new era of customized yet rapidly deployable cancer therapy, combining the precision of engineered cells with the ease of manufacture on a large scale.
Practical Implications of the Research
If these immune-evasive CAR-NK cells prove to be safe in human trials, they would revolutionize the therapy of cancer. Off-the-shelf NK cell therapies would make cutting-edge immunotherapy more effective, cheaper, and available on a broader scale. Patients would no longer have to wait for weeks for their own customized treatment, and immunocompromised patients could also enjoy its advantages.
The strategy may also be applied outside of oncology. The same stealth technology could be used to treat autoimmune diseases such as lupus, or even to develop universal donor cells for regenerative medicine, minimizing transplant rejection.
Over time, this technology might translate cell-based therapies from the research lab to the clinic for many more millions of people.
Research findings are available online in the journal Nature Communications.
Related Stories
- Scientists re-engineer a patient’s own T cells to target and destroy cancer cells
- Breakthrough stem cell therapy could provide permanent cancer immunity
- Scientists create tumor immune hubs to stop cancer growth and prevent relapse
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joseph Shavit
Writer, Editor-At-Large and Publisher
Joseph Shavit, based in Los Angeles, is a seasoned science journalist, editor and co-founder of The Brighter Side of News, where he transforms complex discoveries into clear, engaging stories for general readers. With vast experience at major media groups like Times Mirror and Tribune, he writes with both authority and curiosity. His writing focuses on space science, planetary science, quantum mechanics, geology. Known for linking breakthroughs to real-world markets, he highlights how research transitions into products and industries that shape daily life.