New breath sensor detects diabetes in minutes
New zinc oxide and graphene sensor detects diabetes through breath in minutes, offering a painless and low-cost alternative to blood tests.
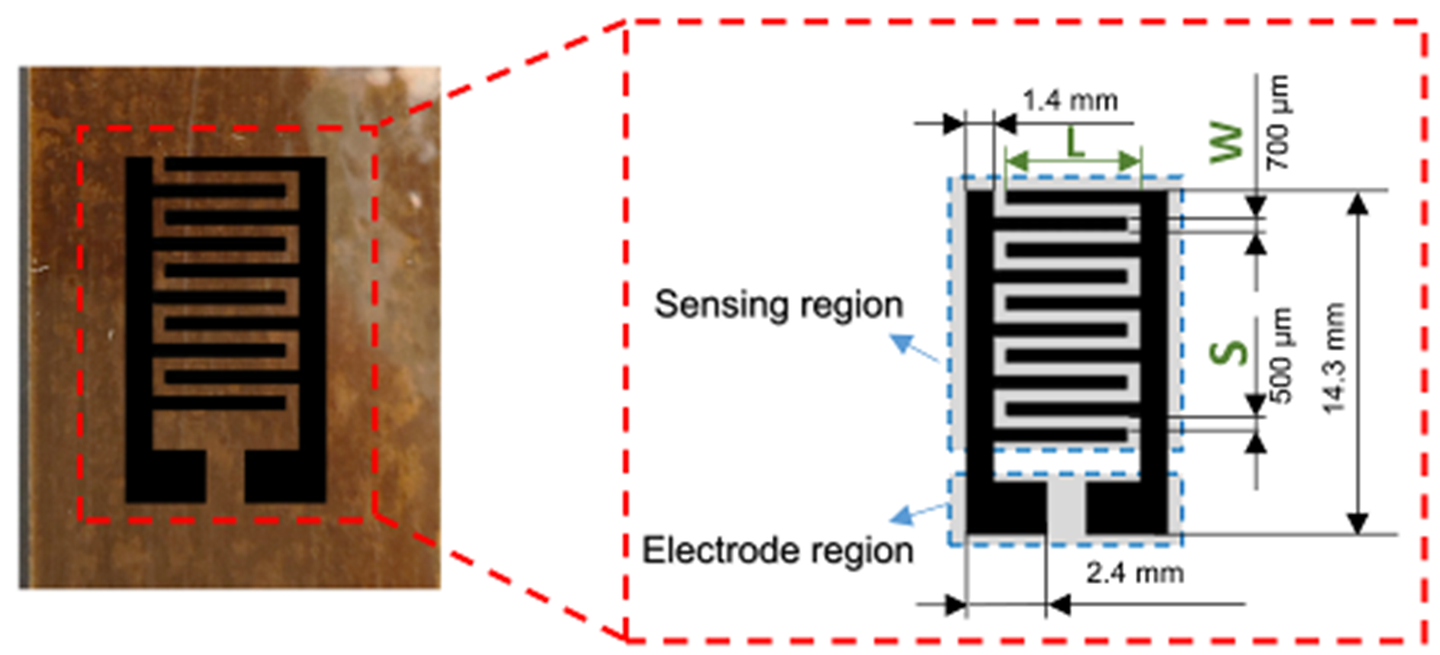
LIG interdigital electrode on the PI film with geometric dimensions shown in the inset. (CREDIT: ScienceDirect)
Acetone may be best known as a sharp-smelling solvent found in nail polish remover, but inside your body it can tell an important story about health. In fact, the amount of acetone in your breath can act as a powerful signal for diabetes. Normally, people exhale only small traces — anywhere from 300 to 900 parts per billion. But when diabetes is present, those levels can rise to more than double that amount. Detecting this invisible gas has long been a goal of medical researchers searching for a painless, reliable way to monitor the disease.
Diabetes affects millions worldwide and is one of the leading causes of death, taking more than six million lives each year. In the United States, more than 37 million adults live with the condition, and one in five do not know it. Early detection and monitoring are crucial, but the most common tests today involve finger pricks, lab visits, or other invasive steps that many find inconvenient. A simple breath test could change that.
Why Breath Matters
Your breath contains hundreds of compounds called volatile organic compounds, or VOCs. Among them, acetone stands out as one of the clearest markers for diabetes. Traditional machines used to measure breath acetone, such as gas chromatography–mass spectrometry or proton transfer reaction mass spectrometry, are highly accurate but also expensive, bulky, and impractical for regular testing.
That challenge has pushed scientists to design portable, low-cost sensors capable of detecting acetone in real time. If successful, such devices could make diabetes testing as easy as breathing into a sensor instead of pricking your skin.
Building Smarter Sensors
Many research groups have turned to gas sensors to solve this challenge. These devices detect gases by analyzing how they interact with sensitive materials. Metal oxide semiconductors, or MOS, are common in this field. Materials like tin oxide, zinc oxide, and titanium oxide respond well to gases but usually need high heat to work properly, which increases energy use.
Carbon nanomaterials such as graphene and carbon nanotubes offer another path. They can function at room temperature, but on their own they lack enough active sites to strongly attract acetone molecules. Combining MOS materials with carbon-based structures, however, gives the best of both worlds — sensitivity and energy efficiency.
Related Stories
- Breath test for blood cancer shows promise in first-ever human study
- First-ever smart face mask can detect kidney disease by analyzing your breath
Zinc oxide, or ZnO, has shown strong results when paired with carbon materials like graphene. Its nanospheres create wide surface areas where gases can move and react, while graphene delivers excellent conductivity. Past experiments with ZnO and reduced graphene oxide or nickel oxide improved performance but often required complex preparation and still struggled to focus specifically on acetone over other gases.
A New Approach with Laser-Induced Graphene
A recent study led by Huanyu “Larry” Cheng, the James L. Henderson, Jr. Memorial Associate Professor of Engineering Science and Mechanics at Penn State, has unveiled a promising breakthrough. The team created a sensor that merges ZnO nanospheres with laser-induced graphene, or LIG. The design is fabricated directly on electrodes using laser direct writing and a simple drop-casting process.
Cheng explains that the method is a bit like toasting bread. By shining a CO₂ laser on a polyimide film, the carbon material transforms into a porous graphene structure. Adjusting the laser’s power and speed can fine-tune the outcome. This porous form allows gases to flow through easily and increases the chance of capturing acetone molecules.
To improve performance, the researchers added a dispersant called sodium dodecyl sulfate to distribute ZnO particles more evenly on the graphene. They also coated the sensor with a molecular sieve known as SBA-15 to protect it from the moisture in breath, which often interferes with gas detection. The sieve’s tiny pores allow acetone to pass while blocking water molecules, making the sensor more reliable under humid conditions.
How the Sensor Works
The strength of the ZnO/LIG sensor comes from the way the two materials interact. ZnO is a semiconductor with a band gap of 3.3 electron volts, while LIG has a band gap of 4.7 electron volts. Together, they form a p–n heterojunction, a type of junction that controls how electrons move.
Here’s what happens during detection: in clean air, oxygen molecules attach to the ZnO surface and capture electrons, which increases resistance. When acetone enters the picture, it reacts with the oxygen, releasing the trapped electrons. This lowers resistance, producing a signal the device can measure. The LIG amplifies the effect by acting as an electron acceptor, which boosts sensitivity and speeds up detection.
The result is a fast, strong response to acetone. In tests, the device detected concentrations as low as 4 parts per billion, far below the typical amounts found in people with diabetes. The sensor reacted to 1 part per million of acetone in just 21 seconds and recovered in 23 seconds, an impressive speed for real-time monitoring.
Human breath is filled with moisture, which often clogs gas sensors or throws off their readings. Without protection, the ZnO/LIG sensor’s performance dropped sharply at high humidity. Adding the SBA-15 coating solved this issue, allowing acetone to pass through while keeping water out. Although the response time slowed somewhat — to about one minute for detection and recovery — the sensor still performed well enough for medical use. It also remained stable for more than two months of continuous testing.
Real-World Testing with Patients
To move beyond the lab, the researchers tested the sensor with real breath samples. They collected samples from 51 people with type 2 diabetes and 20 healthy volunteers. The results were clear: healthy individuals had acetone levels below 1.8 parts per million, while diabetic patients exceeded that threshold. The sensor could easily tell the two groups apart, with responses above 15% in diabetic patients and below that in healthy ones.
Even more encouraging, the sensor’s readings matched closely with fasting blood glucose levels, suggesting it could do more than diagnose diabetes. It could also help track treatment progress. Instead of pricking your finger multiple times a day, you could check your condition by simply breathing into a portable device.
While the current method still requires exhaling into a bag to prevent interference from airflow, Cheng and his team are working to make the sensor even more user-friendly. Future designs may allow direct measurement under the nose or inside a face mask, using condensation from breath. Cheng also sees potential beyond diabetes.
“If we could better understand how acetone levels in the breath change with diet and exercise, in the same way we see fluctuations in glucose levels depending on when and what a person eats, it would be a very exciting opportunity to use this for health applications beyond diagnosing diabetes,” Cheng said.
Practical Implications of the Research
This new ZnO/LIG breath sensor could transform how diabetes is diagnosed and managed. Its low cost, portability, and non-invasive design make it suitable for use at home, in clinics, or in areas where access to medical care is limited. By eliminating the need for needles and lab visits, it reduces barriers to testing and encourages more frequent monitoring.
Beyond diabetes, the sensor’s design could inspire new ways to track health through breath analysis. Since breath contains many compounds linked to different conditions, the same approach could one day help detect other diseases. For now, the work represents a major step toward making life easier for millions living with diabetes.
Research findings are available online in the journal Science Direct.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.