New DNA tool detects ‘zombie cells’ linked to Alzheimer’s and arthritis
Mayo Clinic scientists have developed DNA aptamers that can spot aging cells, opening new doors for treating age-related diseases.

 Edited By: Joseph Shavit
Edited By: Joseph Shavit
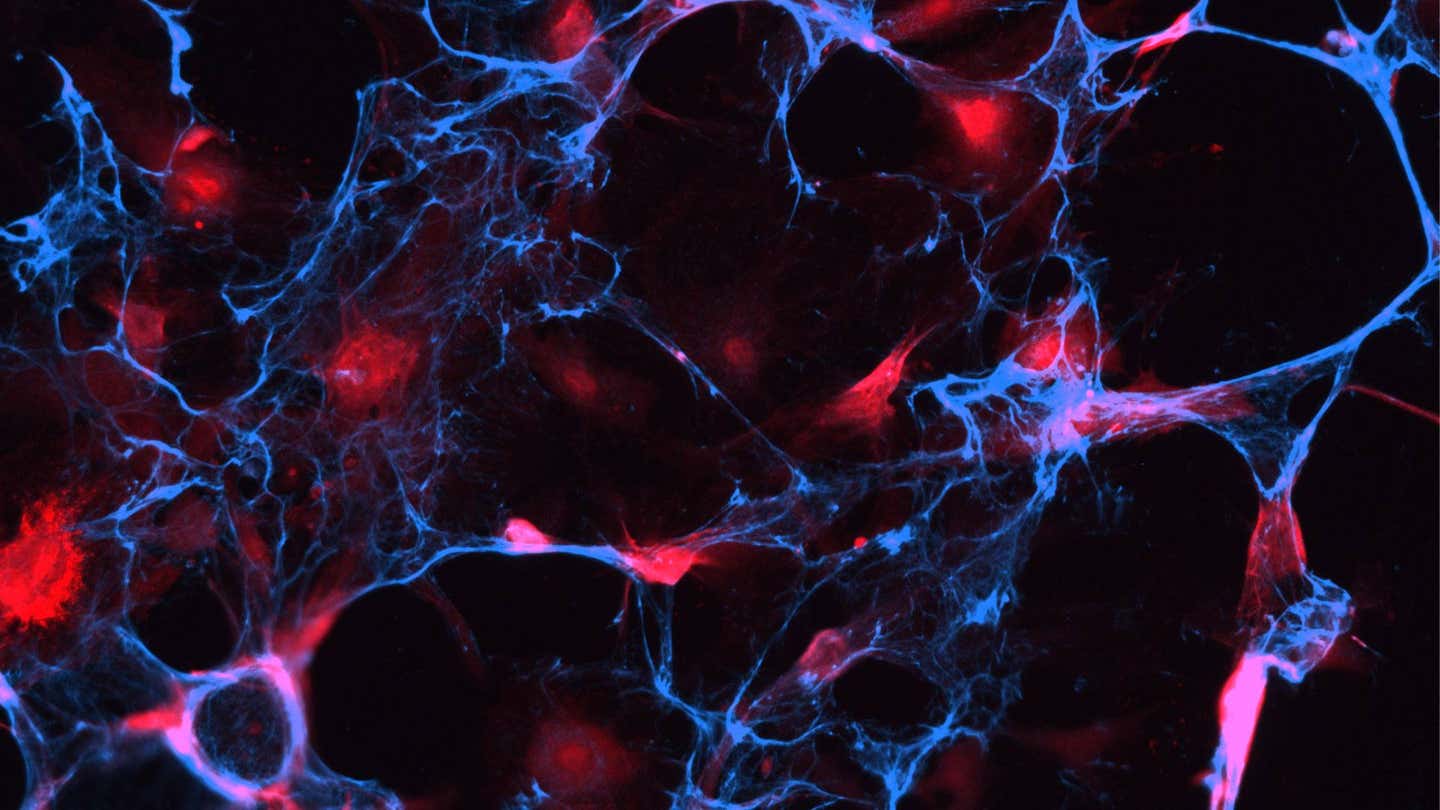
A confocal microscopy image shows senescent cells (red) tagged with molecules known as aptamers (blue). (CREDIT: Aging Cell)
At times, aging doesn't become evident in wrinkles or in gray hair, it occurs deep inside your body, in cells that have ceased to divide but are still alive. These "zombie cells," or senescent cells for short, lie in wait within tissues, excreting toxic molecules that inflame and damage the neighboring cells. In time, their accumulation contributes to maladies ranging from arthritis to Alzheimer’s disease.
For decades, scientists have searched unsuccessfully for a reliable method to detect and clean up senescent cells; however, researchers at the Mayo Clinic may have identified an answer in strands of synthetic DNA.
Breaking the Aging Code
Under the leadership of Keenan S. Pearson and L. James Maher III, researchers at the Mayo Clinic developed tiny DNA tools called aptamers that can accurately identify aged cells in mice. The article documenting their study, published in Aging Cell, can change how researchers will conduct aging studies and treat aging-related diseases.
Aptamers are short nucleic acid sequences that can fold into structures capable of binding specific proteins. They act just like a key fits into a lock.
In their investigation, the Mayo team employed an approach called SELEX (which stands for Systematic Evolution of Ligands by EXponential enrichment) in which they thoroughly searched more than 100 trillion random DNA sequences for bindings to senescent cells. Unlike traditional efforts where a known senescence marker was the starting point, in the SELEX approach, cells themselves would "choose" which DNA easily bound to them.
How the Experiment Worked
To initiate their study, the researchers used fibroblasts, a type of skin cell, from mice to grow in culture. A portion of the subjects was administered a drug that produces DNA damage and induces them to go into senescence. The authors then presented them with their enormous DNA library and monitored which sequences adhered to the senescent cells but not to the healthy cells. After a number of cycles of testing and improvement, they isolated the two aptamers labeled 6756 and 6762, which could repeatedly identify senescent cells.
The investigators further developed the aptamers against additional murine cells, such as liver cells and muscle cells, where the aptamers still served as reliable markers to identify senescent cells. Importantly, they even resolved senescence resulting from radiation or chemical distress.
The authors discovered that the aptamers, when encountering human cells, did not bind to the same targets, which would require the aptamer to be re-engineered to be applicable in human biology. Ultimately, this study represents a tremendous step forward because it is the first report that provides an opportunity to identify where senescent cells hide and how these cells behave in accommodating tissues.
A Fortuitous Student Collaboration
Contrary to the typical high-tech meetings with postdoctoral fellows, the original idea started as a casual conversation between the students. Keenan Pearson, pursuing his graduate degree with aptamers in Dr. Maher's laboratory, bumped into fellow graduate student Sarah Jachim, who conducted aging research under the supervision of Dr. Nathan LeBrasseur. Pearson proposed the idea of using aptamers to identify and characterize senescent cells, and Jachim knew how to handle and culture those cells for assays.
The graduate student's mentors, including Dr. Darren Baker, who investigate the use of therapies directed at targeting senescent cells, were supportive but uncertain about the student's hypothesis; however, Dr. Maher, said, “We frankly loved that this was based on student hypothesis and was a real synergy of two research areas."
What began as a "crazy idea," soon became a cross-lab collaboration that engaged not just the undergraduate labs but also other graduate students to try some microscopy methods and analyze tissues. Within a few short months, the researchers had results showing that aptamers could detect senescent mouse cells with high specificity.
Uncovering the Molecular Target
After they were sure the aptamers had worked, the next question to tackle was, What were they sticking to? Using a sensitive method to analyze the proteins, the team discovered that both aptamers were binding to fibronectin, which is an extracellular matrix protein that helps provide a scaffolding structure around tissues and cells. More specifically, they were binding to a unique variant of fibronectin (FN-EDA1), which is localized in aging or damaged tissues.
Fibronectin itself helps cells hold in place and heal after injury, but too much fibronectin can signal scarring and stiffness in tissues. The aptamers recognizing those changes from the variant proteins mean they are not just detecting the cells, they are detecting that the aging process has changed the tissues and cells.
When researchers tested aptamer 6762 on the lung tissue of mice of different ages, the results were shocking. Young mice had almost no signal, indicating there were senescent cells, while older mice had bright fluorescent staining, which revealed clusters of aging cells. In genetically engineered mice that can clear senescent cells after being treated with a drug, researchers nearly lost the fluorescent signals. This validated the aptamer's accuracy: it wasn't just sticking to some random protein; it was to the actual signature of biological aging.
Beyond the Microscope - What the Results Tell Us
Observing senescent cells is more than a scientific breakthrough; it's a glimpse of the way you change as you age. These cells do not just stop dividing, but they also change their local ecosystem. They secrete proteins that stiffen tissues and start inflammation, and even disrupt other cells nearby.
However, what the Mayo team found is that even once these senescent cells die or relocate, they leave traces behind, changed pieces of fibronectin and collagen that pertain to the site where they were. These marks may shed light on why aging tissues remain stiff and inflamed long after the inciting cells are gone.
"It's an exciting new way to define what it means for a cell to be senescent," said Dr. Maher. "Our method was open-ended. We never told the aptamers what to look for because we wanted them to find what was relevant."
From Discovery to Hope
For individuals suffering from diseases related to aging, for example, fibrosis, diabetes, and neurodegeneration, this research holds promise. Aptamers are cheaper and easier to produce than antibodies, and they can be engineered to deliver therapies directly to cells. In the near future, physicians may be able to conduct aptamer-based tests to detect biological aging in living tissues or deliver medications that eliminate these senescent cells but spare the healthy ones.
Dr. Pearson believes that because of its simplicity, it will ultimately be the most valuable. "Aptamers are adaptable," he said, "and may one day be developed to identify senescent cells in humans, thereby creating a means of intervening at the cellular level to alter the course of aging."
Practical Impact of the Research
This Mayo Clinic study represents a significant move toward understanding and perhaps controlling the biological phenomenon of aging.
Once scientists can identify aptamers that recognize senescent cells, it may be possible to track the biological aging process across tissues, develop diagnostic tests to assess "cellular age," and develop new therapies that either remove these cells or repair them. All of which can help delay the onset of manifest diseases related to chronic tissue inflammation and breakdown.
Working beyond medicine, the end product of this research contributes to our capacity to understand aging on a cellular level and has the potential to slow the biological clock itself.
Research findings are available online in the journal Aging Cell.
Related Stories
- CAR T cells can be reprogrammed to slow down and reverse aging, study finds
- New protein discovery opens the door to slowing or even reversing cellular aging
- Breakthrough anti-aging drug could prolong human life by 30%
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Rebecca Shavit
Writer
Based in Los Angeles, Rebecca Shavit is a dedicated science and technology journalist who writes for The Brighter Side of News, an online publication committed to highlighting positive and transformative stories from around the world. Her reporting spans a wide range of topics, from cutting-edge medical breakthroughs to historical discoveries and innovations. With a keen ability to translate complex concepts into engaging and accessible stories, she makes science and innovation relatable to a broad audience.



