New organism with virus-like traits redefines what it means to be alive
A tiny archaeon discovered inside plankton blurs the boundary between living cells and viruses, challenging how science defines life.
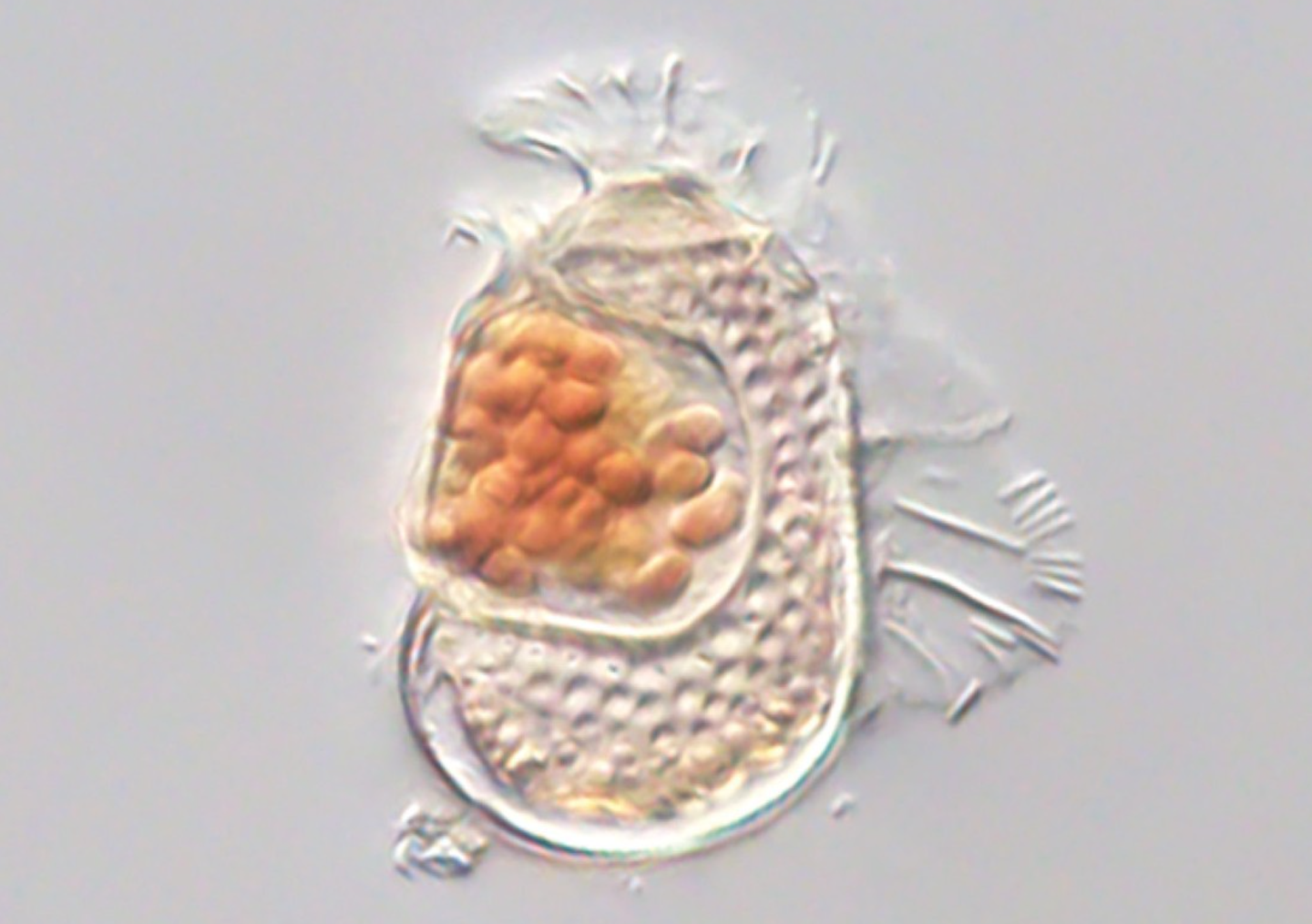
A newly discovered archaeon with a genome smaller than some viruses is redefining the limits of cellular life. (CREDIT: Takuro Nakayama / University of Tsukuba)
In the vast and often unseen world of microscopic life, a recent discovery may force scientists to rethink what it means to be alive.
Nestled inside a tiny plankton cell, researchers found a remarkably small organism unlike anything seen before. Named Candidatus Sukunaarchaeum mirabile, this strange microbe has such a stripped-down genome that it challenges the long-held distinction between living cells and viruses.
The discovery was made by Ryo Harada and his team at Dalhousie University in Canada. While sequencing the DNA of a marine plankton and its symbiotic bacteria, the scientists stumbled upon a genetic sequence that didn’t match any known organism.
What they found was a member of the Archaea domain with a genome just 238,000 base pairs long—less than half the size of the smallest archaeal genome previously known.
A Genome Barely Alive
Sukunaarchaeum’s genetic material focuses almost entirely on copying itself. It carries the machinery for DNA replication, transcription, and translation—key tasks needed to pass on its genetic code. However, it lacks nearly every other known pathway for metabolism or energy production. That means it can’t make its own food, build its own parts, or power itself. Instead, it depends entirely on its host to do all the heavy lifting.
Viruses also rely on hosts, but they go a step further: they don’t carry genes for basic cell functions like building proteins. Sukunaarchaeum is different. It still has the ability to create ribosomes and messenger RNA—crucial tools for making proteins. That distinction sets it apart and keeps it, at least for now, within the realm of cellular life.
“This extreme specialization challenges our fundamental understanding of the minimal requirements for cellular life,” wrote Harada and his team in their paper posted to bioRxiv.
Related Stories
A New Branch on the Tree of Life
Scientists classify all life into three major domains: Bacteria, Archaea, and Eukaryota. Archaea are single-celled organisms that often live in extreme environments. Sukunaarchaeum belongs to this group but forms a deeply branching and previously unknown lineage. Its genes reveal ties to other ancient microbes, but it also represents a distinct new branch that had been hidden from scientific view.
The discovery came from analyzing environmental DNA taken from a single dinoflagellate cell. These cells are a type of plankton and are known to host many microbes on or inside them. Using a method called single-cell genome amplification, researchers were able to capture and sequence the tiny circle of DNA belonging to Sukunaarchaeum.
Its presence inside another organism, paired with its extreme genetic dependence, suggests it lives a parasitic lifestyle. Yet it doesn’t appear to provide any clear benefit to its host. It may be one of the most extreme examples yet of symbiosis—or parasitism—among microbes.
The World of Ultra-Reduced Genomes
This discovery fits into a growing body of research focused on microbes with very small genomes and unusual lifestyles. Groups like the Candidate Phyla Radiation (also known as Minisyncoccota) and the DPANN archaea are known for having rapid genetic changes, limited metabolic abilities, and strong dependence on their hosts.
These microbes often can't be grown alone in the lab. They survive only when paired with other cells that provide the materials and energy they lack. Cultured examples of these groups have shown that many live in tight symbiotic relationships, often exchanging nutrients and chemical signals.
Sukunaarchaeum may push this dependency even further. Unlike others that retain some metabolic genes, this archaeon appears to rely entirely on its host for survival. It has no genes for producing energy or nutrients. Its only priority seems to be reproduction.
“This suggests an unprecedented level of metabolic dependence on a host,” the researchers said, “a condition that challenges the functional distinctions between minimal cellular life and viruses.”
Blurring the Line Between Virus and Cell
For years, scientists have debated whether viruses are truly alive. Viruses can't replicate or make proteins on their own. Instead, they hijack the machinery of their host cells to reproduce. Because of this, many researchers classify them as non-living biological entities.
Sukunaarchaeum complicates that idea. It can make the proteins it needs to copy its DNA. It can build some of its own structures, possibly even a membrane to contain its genome. But it lacks the tools to keep itself alive outside of a host. This places it in a gray zone between life and non-life.
Interestingly, some viruses have larger genomes than Sukunaarchaeum. Giant viruses can carry up to 2.5 million base pairs, while this archaeon survives with less than 240,000. Yet Sukunaarchaeum still manages to perform key cellular functions that viruses can't.
Its discovery reveals just how blurry the definition of life can be. While it falls short of being a fully independent organism, it still meets some of the criteria used to define living cells.
A Hidden Frontier of Microbial Life
The ocean is full of single-celled eukaryotes, many of which host a variety of microbial symbionts. These host-microbe relationships are proving to be rich sources of biological novelty. In fact, scientists are beginning to uncover new cellular structures, such as the chromatophore in Paulinella and the nitroplast in Braarudosphaera bigelowii, that evolved from prokaryotic symbionts.
These discoveries suggest that the surfaces and insides of tiny marine organisms may hold many more surprises. Symbiotic microbes like Sukunaarchaeum might be common but have remained hidden due to their tiny size and the challenges of studying them.
As genomic tools improve, scientists are finding that many of the smallest and most reduced cells are deeply intertwined with other organisms. Whether they offer help, steal resources, or simply tag along, these microscopic passengers could reshape how we understand life’s complexity and evolution.
Sukunaarchaeum’s genome, focused entirely on reproduction and survival within a host, represents a new extreme in this spectrum. It may not be the only one. The more scientists look inside these microbial partnerships, the more likely they are to find other life forms that break the rules.
This tiny archaeon with its minimalist genome stands as a reminder that biology is full of exceptions. Life, it seems, is not always as clear-cut as textbooks suggest. Even in the smallest cells, nature finds ways to blur the lines and rewrite the rules.
The research paper has been uploaded onto bioRxiv and has yet to be peer reviewed.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.



