Novel device measures nerve activity to successfully treat sepsis and PTSD
A team of engineers and physicians at UC San Diego has developed a device to non-invasively measure cervical nerve activity in humans.
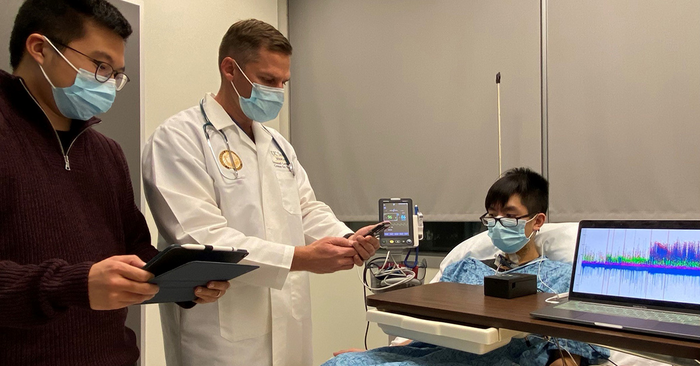
[Dec 7, 2022: Nicole Mlynaryk, University of California - San Diego]
UC San Diego researchers Yifeng Bu and Imanuel Lerman test an array of electrodes placed on the neck that can document electrical activity in the brain related to stress. The new tool could help diagnose patients whose medical conditions are particularly vulnerable to increased levels of stress. (CREDIT: UC San Diego)
A team of engineers and physicians at University of California San Diego has developed a device to non-invasively measure cervical nerve activity in humans, a new tool that they say could potentially inform and improve treatments for patients with sepsis, a life-threatening response to infection, and mental health conditions, such as post-traumatic stress disorder (PTSD).
The device is described in Scientific Reports.
“For the first time, we have identified cervical electroneurographic evidence of autonomic (fight or flight versus rest and digest) biotypes that are remarkably consistent across different challenges to the autonomic or involuntary nervous system,” said senior author Imanuel Lerman, MD, clinical professor of anesthesiology at UC San Diego School of Medicine, with additional appointments at the UC San Diego Qualcomm Institute, UC San Diego Jacobs School of Engineering and the VA Center of Excellence for Stress and Mental Health.
The research builds upon the numerous and fundamental roles of the cervical vagus nerve, the upper portion of the vagus nerve that runs from the gut to the brain, delivering information on the status of surrounding inner organs, overseeing crucial bodily functions like immune response and digestion and playing a role in major psychiatric conditions, such as mood and anxiety disorders.
Related Stories:
The new device features a flexible array of electrodes that stretch from the lower front to the upper back of the neck, allowing researchers to capture electrical activity across different nerves. An integrated user interface permits real-time visualization of data and a custom algorithm groups persons according to their nervous systems’ response to stress.
In the past, measuring nerve activity in the neck often called for surgically implanting microelectrodes. Lerman, with co-author Todd Coleman, PhD, a professor in the Department of Bioengineering at UC San Diego Jacobs School of Engineering, set out to create a less risky and less invasive method by adapting existing technology Coleman had developed with colleague Jonas Kurniawan, PhD, a postdoctoral researcher at Stanford University. The resulting flexible array can be worn for up to a day and moves easily with the patient’s head and neck movements.
To explore human autonomic biotypes, or groups of patients whose involuntary nervous systems responded similarly to stress, the researchers ran a series of tests that asked study participants to place and hold their hand in ice water, followed by a timed breathing exercise. The array recorded cervical nerve signaling or cervical electroneurography, including heart rate in subjects before and after both the ice water challenge and during the breathing exercise.
Researchers found that study participants fell consistently into two distinct biotype groups: those whose neural firing and heart rates increased during both tests and those who exhibited the opposite trend. The device’s unique algorithm identified differences in the response of specific nerve clusters to stressors, such as pain induced by the ice water, and physical symptoms, such as sweating and increased in heart rate associated with the timed breathing challenge.
“The results are exciting,” said Coleman. “The array was capable of recording autonomic nervous system activity, and we were pleasantly surprised to observe consistent autonomic response across stress test challenges. More work is needed, however, to demonstrate our sensor capabilities in larger populations.”
Custom surface electrode array for non-invasive testing of cervical neuronal activity. (a) Device cross section (5 mm sensor diameter and 500 μm interconnect width). (b) Cleanroom post-processed wafer ready to be printed with Ag/AgCl ink. (c) Ag/AgCl ink screen-printed wafer on the active electrode region. (d) Device transfer-printed onto a self-adhering flexible silicone substrate (Ecoflex/Silbione) on PET backing via water soluble tape. (CREDIT: Scientific Reports)
Although the electrode array could not identify the precise nerves firing in response to the stress and pain of the cold-water challenge, the researchers said they hope it will someday aid in diagnosing and treating conditions like PTSD and sepsis.
For example, the vagus nerve triggers inflammation in response to injuries or infection in the body, a mechanism that can become disrupted with PTSD. The authors said their new device might eventually assist clinicians in measuring patients’ response to therapy for PTSD, such as deep breathing exercises employed during mindfulness meditation, by monitoring neural firing in the vagus nerve.
The custom design allows for free movement without distorting the adhesiveness and robustness of the physical structure of the electrode array. (a) The custom surface electrode array is adhesive integrated, flexible, and non-invasively attached to the subject's anterior cervical neck, placed lateral to the trachea and medial to the sternocleidomastoid. (CREDIT: Scientific Reports)
Lerman is already one of a number of researchers using electrical vagus nerve stimulation to test whether stimulating these neural structures can decrease inflammation and pain in people with PTSD.
The array could also promote safety in pilots operating military aircraft by detecting flares in nerve activity that prompt dizziness or nausea.
Within hospital settings, the authors suggest, the device might help flag patients susceptible to life-threatening conditions like sepsis by identifying people who react strongly to physical stress.
Sepsis occurs when the body’s immune system overreacts to an infection, damaging its own tissues in the process. Mortality risk rapidly increases with time, so technology that aids in the detection and flagging of at-risk hospitalized patients would provide physicians with an early warning to administer antibiotics, improving a patient’s chances of avoiding or surviving sepsis.
As a next step, researchers plan to integrate the array with additional hardware for a wireless, wearable sensor that can be deployed outside the laboratory. The researchers are now developing plans for an in-hospital sepsis detection clinical trial.
The study was a cross-disciplinary effort involving researchers at the UC San Diego Qualcomm Institute, UC San Diego School of Medicine, UC San Diego Jacobs School of Engineering (Electrical and Computer Engineering, Materials Science and Engineering, NanoEngineering and Bioengineering), the Department of Physics and the Herbert Wertheim School of Public Health and Human Longevity Science, plus faculty at Stanford University and the VA San Diego Healthcare System.
Co-authors include: Yifeng Bu, Jonas F. Kurniawan, Jacob Prince, Andrew K.L. Nguyen, Brandon Ho, Nathan L.J. Sit, Timothy Pham, Vincent M. Wu, Boris Tjhia, Tsung-Chin Wu, Xin M. Tu and Ramesh Rao, all at UC San Diego; and Andrew J. Shin, Stanford University.
Note: Materials provided above by University of California - San Diego. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.



