Researchers discover genetic cause of hearing loss and identify possible treatments
Scientists have uncovered how mutations in the CPD gene cause hearing loss—and how arginine or nitric oxide–boosting drugs could restore hearing.
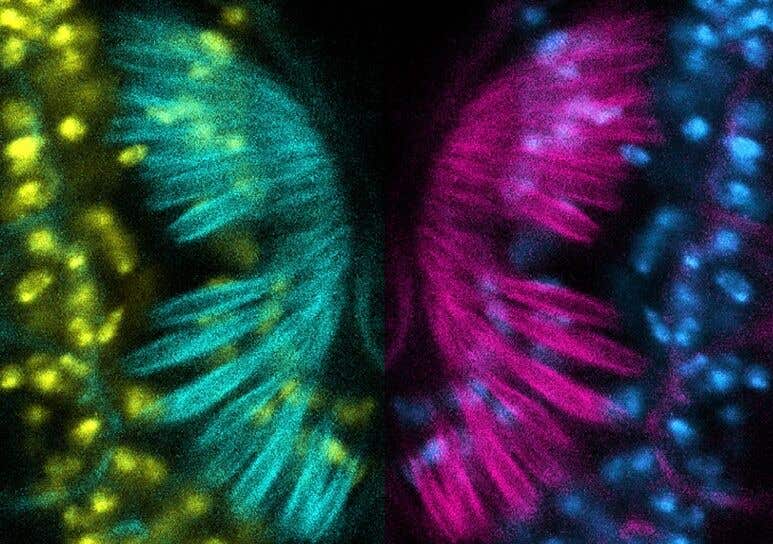
Fruit fly models of deficiency in a gene called CPD show defects in Johnston’s organ (JO), a sensory organ on the antennae that helps sense gravity, wind flow, and near-field sound. This confocal micrographs image displays immunolabeled structures within the cilium of the JO. (CREDIT: Natalie Ortiz-Vega, Rong Grace Zhai)
Researchers have understood for decades that some forms of deafness are inherited, yet until now, few of those genetic clues have led the way to therapy. That is beginning to change.
An international team of researchers led by Rong Grace Zhai from the University of Chicago and Memoona Ramzan and Mustafa Tekin at the University of Miami identified a gene mutation that causes severe congenital hearing loss. Unexpectedly, they also found that the condition may be treatable. The treatment could involve something as simple as a nutritional supplement.
Their study targets mutations in a gene called carboxypeptidase D (CPD). These abnormal genetic mutations disrupt nitric oxide signaling in the inner ear, damaging the delicate hair cells that detect sound. By supplementing this pathway with amino acid supplements like L-arginine, or with drugs such as sildenafil (Viagra), the researchers found they could improve hearing-related function in animal and cell models.
The Gene Behind the Noise
The CPD gene belongs to a family of enzymes known as metallocarboxypeptidases, which modify proteins by trimming their ends. Though CPD is active throughout the body, its role in hearing was not known until now. The research discovered that mutations in the gene's second catalytic domain interfere with the synthesis of arginine—an amino acid that is vital to synthesize nitric oxide.
Nitric oxide is a cellular messenger and protector of sorts, particularly in the cochlea, the spiral-shaped structure that receives sound. When nitric oxide levels decrease, sensory hair cells within the cochlea are exposed to stress and damage. Once hair cells die, they do not regenerate and hearing loss becomes permanent.
"This study is exciting since we found a new gene mutation related to deafness—and, more importantly, we discovered a therapeutic target that might actually be useful," said Rong Grace Zhai, Ph.D., Jack Miller Professor for the Study of Neurological Diseases at the University of Chicago.
Three Families, One Common Mutation
The discovery began with a genetic analysis of 1,012 families with sensorineural hearing loss (SNHL)—deafness resulting from inner ear or auditory nerve damage. After ruling out previously discovered genes, three unrelated Turkish families stood out. All had children who were born with severe, bilateral hearing loss but no other symptoms.
Sequencing revealed rare mutations in CPD that altered single amino acids in the enzyme so that it was less functional. In one family, the variant was c.1688T>G (p.Met563Arg); in others, c.2498G>A (p.Arg833His) and c.2372A>G. The mutations interfered with the function of the enzyme in regulating arginine metabolism, impairing the production of nitric oxide and rendering the sensory cells of the cochlea susceptible.
Remarkably, despite the fact that CPD is active in many organs, its removal seemed to have an effect on the cochlea only. Researchers believe that this organ lacks the backup pathways other tissues use to compensate for disrupted nitric oxide signaling, making it especially susceptible to metabolic disruption.
Testing the Pathway in Mice and Flies
To confirm their findings, the scientists tested CPD mutations in mouse models, human-derived cells, and fruit flies. CPD loss in mice led to oxidative stress and cell death in the sensory hair cells of the cochlea. Fruit flies without the CPD gene had hearing and balance impairment and difficulty with basic movement.
When L-arginine was administered to these models, nitric oxide and cyclic GMP (cGMP) levels normalized to rescue cells from death. Even more striking, flies treated with L-arginine or sildenafil had improved behavior, reflecting partial restoration of auditory function.
It turns out that CPD maintains the arginine level in the hair cells so that fast signaling can take place through nitric oxide," Zhai said. "Those cells are particularly vulnerable when CPD is gone because they use that pathway to stay alive."
Hope Beyond the Lab
For the majority of individuals born with deafness, the choices are cochlear implants or hearing aids—implants that may aid in the detection of sound but do not restore natural hearing. The CPD finding defies that expectation by recognizing a molecular pathway that might be addressed with medication or diet.
Sildenafil, best known for its treatment of erectile dysfunction, works by enhancing the same nitric oxide–cGMP pathway disrupted by CPD mutations. That implies, along with the L-arginine's therapeutic effects in fruit flies, that drugs already on the market for other conditions can be repurposed for genetic hearing loss.
"What's important about this is that not only do we understand the mechanism of this form of deafness, but we also found a promising therapeutic avenue," Zhai said. "It's a nice example of how FDA-approved compounds can be repurposed to treat orphan diseases."
Why Nitric Oxide Matters:
Nitric oxide might sound like a substance in a chemistry lab, but in your body, it plays a very significant role. It causes blood vessels to relax, regulates immune response, and protects neurons from damage. In the ear, nitric oxide activates the cGMP-Prkg1 pathway, which protects sensory cells from stress and aging.
In cells that do not have CPD, this system collapses, leaving the cochlea vulnerable. L-arginine treatment restores nitric oxide, resuming this protective signaling. The research suggests that nitric oxide's importance in the ear may extend beyond birth defects, with a possible role in age-related hearing loss too.
A Rare Disorder With Far-Reaching Meaning
While CPD-related hearing loss is unusual, the discovery has broader implications. Nitric oxide signaling is important in nearly every organ system, from the heart to the brain. Understanding how CPD maintains arginine levels could shed light on research into cardiovascular health, immune diseases, and neurodegenerative conditions.
Because CPD knockout mice are not viable, the scientists believe that complete loss of the enzyme would be fatal in humans. But the Turkish families' partial loss reveals how specific tissues—the cochlea, for instance—can become the Achilles' heels of an otherwise very strong system.
Practical Implications of the Research
This research opens up a new avenue for the treatment of genetic deafness. By identifying a pathway that can be treated using nutritional supplements or medications that are already available, researchers have shown that genetic hearing loss does not have to be irreversible. Follow-up studies will test arginine and nitric oxide–enhancing therapies in mammals to determine safe dosages, side effects, and long-term results.
If successful, these findings would result in the creation of the first pharmacological treatment for inherited deafness, offering new hope for restored hearing for millions of people.
It also suggests that gene-based disorders in the future can be treated with simple metabolic therapies rather than complicated genetic engineering.
Research findings are available online in the Journal of Clinical Investigation.
Related Stories
- Gene therapy restores hearing in deaf children and adults
- Harvard gene editing treatment corrects brain mutations and improves survival
- Light-based therapy may finally offer relief for millions of tinnitus sufferers
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joseph Shavit
Writer, Editor-At-Large and Publisher
Joseph Shavit, based in Los Angeles, is a seasoned science journalist, editor and co-founder of The Brighter Side of News, where he transforms complex discoveries into clear, engaging stories for general readers. With vast experience at major media groups like Times Mirror and Tribune, he writes with both authority and curiosity. His writing focuses on space science, planetary science, quantum mechanics, geology. Known for linking breakthroughs to real-world markets, he highlights how research transitions into products and industries that shape daily life.