Scientists create tumor immune hubs to stop cancer growth and prevent relapse
Scientists found a way to grow immune hubs inside tumors, stopping cancer growth and preventing relapse in mouse studies.
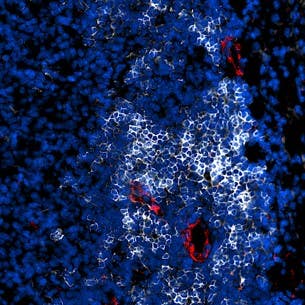
A fluorescence image shows a tertiary lymphoid structure (TLS) inside a tumor, where B cells (white) form dense clusters surrounded by high endothelial venules (red)—specialized blood vessels that recruit B and T cells from circulation into the tumor to mount an immune response against cancer. (CREDIT: Nature Immunology)
Researchers have identified how to encourage tumors to build their own immune hubs—structures that look and function like lymph nodes—deep in the middle of cancer. Such immune hubs, known as tertiary lymphoid structures, or TLS, propel immune cells to destroy tumors more effectively and even stop them from returning. Created in mice, the breakthrough could overhaul the way doctors treat patients with cancers that are normally not sensitive to the immune system.
"Hotting" Up Cold Tumors
All tumors are not equal. Some are "immune hot," meaning immune cells pour in and attack. Those are more likely to be treated with treatments like immunotherapy. Some are "immune cold," mostly invisible to the immune system. Those patients are more likely to have a worse prognosis.
TLS are distinct collections of immune cells that form in tissues as a result of chronic inflammation, including in some cancers. If they form in a tumor, patients survive longer and respond better to treatment. The challenge has been how to get TLS to form in cancers that do not contain them. Earlier attempts made structures look like TLS but did not work entirely.
That's where this new study steps in.
Researchers at Johns Hopkins All Children's Hospital tried out whether the activation of two immune pathways at the same time could trigger TLS in "immune cold" tumors. They hit on STING, a sensor for danger that sounds alarms all over the body, and a receptor called LTβR, which helps build lymphoid tissues.
A Two-Pathway Punch
The scientists injected mice with different types of cancer cells—pancreatic, breast, and muscle tumors. When tumors reached a certain size, they exposed some animals to a STING activator, some to an LTβR-stimulating antibody, and some to both.
The contrast was striking. Tumors exposed to one stimulus barely grew TLS. But when both signals were triggered, the majority of tumors nurtured mature immune centers. These TLS had compact B cell centers surrounded by T cells and characteristic blood vessels called high endothelial venules, which act like portals to usher in new immune warriors from the circulation.
"These findings show that we can induce functional TLS therapeutically in otherwise immune-cold tumors," said Masanobu Komatsu, a senior scientist at Johns Hopkins All Children's Cancer & Blood Disorders Institute. "By building the right immune infrastructure in tumors, we can boost the patient's own defense—both the T cell and B cell arms—against cancer growth, relapse, and metastasis."
Tumors Shrink and Stay Away
The researchers didn't just see TLS form-they saw them work. For mice with pancreatic cancer, the tandem treatment reduced tumor size in half. Breast and muscle tumors also decelerated. Alone, the treatments had minimal effect.
Then came more encouraging news. When scientists treated tumors, removed them surgically, and then re-introduced cancer cells, most mice rejected the new growth. The animals not only survived, but they stayed cancer-free in the long term. The combination treatment successfully "immunized" them, so a memory of the tumor existed so the immune system could overcome it again.
In contrast, mice that were depleted of key immune cells like CD4+ T cells, CD8+ T cells, or B cells lost that immunity. The experiments also showed that long-term tumor control depends on both antibody-secreting B cells and killer T cells working together.
B Cells Lead the Charge
Among tumors treated with the dual therapy, the stars of the show were B cells. They represented more than a quarter of all immune cells present in the tissue. They developed into plasma cells, which produced IgG antibodies designed to be able to target the tumor. Others became long-lived memory B cells, ready to fight if the cancer ever recurred.
Tests confirmed that these antibodies were significant. Serum from treated mice suppressed tumor growth when injected into untreated mice. Without B cells, however, the treatment could not induce lasting protection.
This collaboration between cellular combatants (T cells and natural killer cells) and antibody responses gave the immune system precision and memory. Together, they created a two-barreled assault that could shrink tumors and prevent relapse.
From Lab to Clinic
The research, backed by the National Cancer Institute and other federal programs, is still in its early stages. It has so far only been tested on mice, but the researchers are optimistic. STING activators are already being tested on cancer patients, and LTβR-targeting drugs could be made available for humans. If this approach is successful, it could improve how existing treatments like chemotherapy and checkpoint inhibitors work.
"Booster of T-cell activity early on not only kills tumor cells, but also optimizes the maturation of TLS that support anti-tumor responses," Komatsu said.
The researchers continue to explore the mechanisms of TLS treatment and in anticipation of clinical trials in children and adults.
Practical Implications of the Research
If further human trials confirm these results, the ability to cause TLS within tumors would be a game-changer for cancer treatment. Those patients whose immune-cold tumors currently have limited options may be treated with therapies that make their cancers become hotspots of immunity.
This would not only maximize survival but also minimize the risk of recurrence. By stimulating long-term immune memory, the immune system could potentially fight against recurrence in the same manner that a vaccine inhibits infection. The research could also enhance the versatility of immunotherapy such that treatment is effective for more types of cancer.
In the long run, such strategies might personalize cancer treatment by designing the tumor environment itself to enhance the immune system's battle.
Research findings are available online in the journal Nature Immunology.
Related Stories
- UCLA develops new immune cell therapy to battle deadly kidney cancer
- New AI-driven cancer treatment builds immune defenses in weeks
- New bio-engineered molecule kills cancer cells and activates the immune system
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Mac Oliveau
Writer
Mac Oliveau is a Los Angeles–based science and technology journalist for The Brighter Side of News, an online publication focused on uplifting, transformative stories from around the globe. Passionate about spotlighting groundbreaking discoveries and innovations, Mac covers a broad spectrum of topics including medical breakthroughs, health and green tech. With a talent for making complex science clear and compelling, they connect readers to the advancements shaping a brighter, more hopeful future.