Scientists found the genetic switch that makes primary cilia grow
New MSK research reveals how SP5 and SP8 genes trigger primary cilia growth, unlocking insight into rare diseases called ciliopathies.
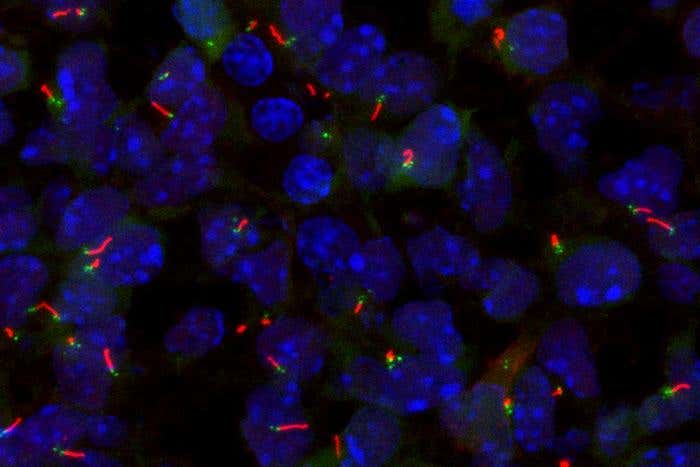
A new MSK study finds that two genes, SP5 and SP8, act as master switches for forming primary cilia — crucial structures in early development. (CREDIT: Memorial Sloan Kettering Cancer Center)
Some see a finger. Others, a worm. Scientists often call it an antenna. This tiny structure, sticking out from the surface of most human cells, is known as the primary cilium. Though nearly every cell has one, for decades, it was left out of textbooks. Now, new research from Memorial Sloan Kettering Cancer Center (MSK) is bringing it into the spotlight.
Primary cilia play a powerful role in how cells sense their surroundings. They help guide embryo development and ensure organs grow in the right place. If they don’t form properly, the results can be serious — from hearing loss to heart defects. Despite their importance, researchers have long wondered: What tells a cell to build one in the first place?
A new study, published in Science, reveals part of the answer. Developmental biologists Dr. Yinwen Liang and Dr. Alexandra Joyner discovered that two genes, called SP5 and SP8, act like on-off switches for primary cilium formation. Their work sheds light on how cells decide to grow this vital structure — and could open new doors for treating a group of disorders known as ciliopathies.
What Are Cilia and Why Do They Matter?
Cilia are hair-like projections found on the surface of cells. There are two main types. Motile cilia, which move fluids around the body, are found in places like the lungs. Primary cilia, by contrast, don’t move. Instead, they work like antennae. They sense signals and help cells respond properly during development.
Each primary cilium is made from hundreds of proteins. Many of these are shared with motile cilia, but each type also has unique parts. If something goes wrong in their structure or function, it can lead to ciliopathies. These are a group of rare genetic disorders that affect about 1 in 2,000 people globally. Symptoms can range from birth defects to kidney disease and even learning problems.
Dr. Kathryn Anderson, a respected scientist at MSK, made a major breakthrough in 2003 when she showed that primary cilia help interpret “Hedgehog” signals — crucial molecular messages during embryo growth. These signals guide how organs form, including the brain and spinal cord. Her discovery pushed the scientific community to pay closer attention to this overlooked organelle.
Still, one question remained: What starts the process of building a primary cilium? That’s where the new study comes in.
Related Stories
- Male embryos grow faster: Breakthrough study reveals genetic reasons why
- New CRISPR breakthrough could transform genetic disease treatment
Finding the Genetic Switch
When Dr. Liang joined the Joyner Lab at MSK, she focused on why some cells build primary cilia and others don’t. Her first theory was that cells lacking cilia might actively break them down. She tested this by trying to block the disassembly process. But the results didn’t change. Blocking disassembly didn’t make the cells build cilia.
So, she and Dr. Joyner took a different approach. Instead of focusing on disassembly, they wondered if certain genes might act as instructions, telling a cell whether or not to build a cilium.
To explore this idea, the team used a powerful tool called single-cell RNA sequencing, or scRNAseq. This method lets researchers see which genes are turned on in individual cells. They looked at mouse embryos because most of their cells grow primary cilia — except one: the yolk sac visceral endoderm (YsVE), an outer layer that supports the embryo but does not form organs.
By comparing gene activity in ciliated versus non-ciliated cells, the researchers identified over 100 genes that were more active in ciliated cells. Among these, they looked for “transcription factors.” These are special proteins that act like switches, turning other genes on or off.
Next, the team used another tool called ATAC-seq. It shows which parts of the DNA are open and readable, a sign that those areas are active. This helped narrow down their search to two genes — SP5 and SP8 — that were highly active in cells with cilia but silent in those without.
What happened next surprised everyone.
Turning Cilia On — With a Single Gene
To test their theory, the scientists removed SP5 and SP8 from cells that normally grow cilia. The result? Far fewer cells grew them, and those that did had shorter, incomplete versions. Then, they did the opposite — adding SP8 to cells that normally don’t grow cilia.
The outcome was striking.
“If you add SP8 to extraembryonic cells, many of the cells now make cilia,” said Dr. Joyner. “No one’s ever seen that result before.”
In other words, SP8 was enough to turn on the cilium-building process in cells that had never made them before. The team believes that SP5 and SP8 are at the top of the decision-making process — they flip the master switch that tells a cell to start building a cilium.
Dr. Liang sees this as a major step forward. “We see it as a big breakthrough to find the upstream transcription factors that switch the whole thing on,” she explained.
These findings were supported by further studies using embryonic stem cells and “gastruloids,” lab-grown clumps that mimic early embryo development. Removing SP5 and SP8 from these models also reduced the number and length of cilia, especially in tissues like the brain and lungs.
The research also revealed that SP5 and SP8 regulate not just primary cilia but also some genes involved in motile cilia — though only SP8 seems capable of starting primary cilia formation by itself.
Broader Impact on Human Health
Ciliopathies are complex disorders. Some patients are born with their heart on the wrong side of the body. Others may experience fluid buildup in the brain (hydrocephalus), infertility, or problems with movement and balance. Because these conditions often affect multiple organ systems, they can be hard to diagnose and treat.
By identifying SP5 and SP8 as master regulators, this study opens new pathways for understanding these diseases. If doctors can pinpoint which gene is malfunctioning in a patient, they might one day be able to restore cilium formation through gene therapy.
This research could also improve how scientists grow stem cells in the lab. Many treatments under development rely on stem cells forming proper tissues. If the right transcription factors are missing, these cells may never function properly. Knowing how to switch on primary cilia could improve the quality of lab-grown tissues and organs.
Dr. Liang, who is starting her own lab in China, hopes to build on this work. “My long-term goal is to improve our understanding of how cilia are formed and then use that information to benefit the clinical study of ciliopathies,” she said.
Dr. Joyner, now retired, spent her career studying how genes shape embryo development. Her final project, this breakthrough study, continues the legacy of her mentor, Dr. Anderson, and shows how even the smallest parts of a cell can hold the biggest answers.
From Mystery to Mechanism
The discovery that SP5 and SP8 control primary cilia formation marks a major turning point in cell biology. These genes are now considered likely candidates in human ciliopathies, though more work is needed to confirm their roles in patients.
The study also hints at a broader genetic network. SP5 and SP8 activate other transcription factors, including some known to regulate motile cilia genes. However, they appear to only be able to trigger the building of primary cilia on their own — not the motile ones.
That distinction matters. It means that while SP5 and SP8 start the process, other genes must join in to build more complex cilia used for movement. By mapping this genetic tree, scientists are starting to understand how cells “decide” what kind of cilia to grow, or whether to grow them at all.
The results may also help explain some developmental mysteries. For example, why certain tissues form normally even when others do not. If SP5 and SP8 are missing or altered in just a few cells, it could lead to specific, localized defects — something doctors often see in ciliopathy patients.
This study shows what’s possible when scientists combine advanced genetic tools with a clear focus. It also reminds us that answers often lie in the overlooked and the microscopic. Once thought too small to matter, the primary cilium is proving to be a key player in the complex symphony of life.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.



