Scientists recreate the universe’s first molecule in the lab
Within minutes of the Big Bang, conditions had cooled enough for the very first atoms—mostly hydrogen, helium, and a touch of lithium—to form.
New research shows helium hydride, the universe’s first molecule, was a powerful coolant that paved the way for star formation after the Big Bang. (CREDIT: W. B. Latter (SIRTF Science Center / Caltech) and NASA)
About 13.8 billion years ago, the newborn universe was a blazing sea of energy and particles. Within minutes of the Big Bang, conditions had cooled enough for the very first atoms—mostly hydrogen, helium, and a touch of lithium—to form. But it wasn’t until much later, when these atoms began linking up into molecules, that the stage was set for stars to flicker into existence.
One of those first molecules was helium hydride, or HeH⁺. For decades, scientists suspected this ion played a critical role in cooling the early cosmos, helping the first gas clouds collapse into stars. A new study published in Astronomy and Astrophysics has now recreated helium hydride’s reactions under conditions that mimic the early universe. The results suggest that this humble ion may have been far more important for star formation than scientists once believed.
Building blocks of the cosmos
At the start, the universe was too hot for atoms to hold onto their electrons. Everything existed as a swirling plasma of charged particles. As space expanded and temperatures dropped, electrons began to “recombine” with atomic nuclei, forming stable hydrogen and helium atoms. This period, called the recombination era, marked the beginning of real chemistry.
Helium was one of the first elements ready to react, and when it combined with hydrogen, it created helium hydride ions—the earliest known molecule. HeH⁺ didn’t stick around for long, but its presence mattered. These ions were precursors to molecular hydrogen, H₂, which is the most common molecule in the universe today and absolutely vital for star birth.
Why? Because molecules can do something single atoms can’t: they can shed heat efficiently. Vibrations and rotations inside molecules let them radiate away energy, cooling down clouds of gas. Without this cooling, those gas clouds could not have condensed enough to ignite nuclear fusion, the engine that powers stars.
A surprising reaction
For years, scientists thought one key reaction involving helium hydride slowed dramatically at lower temperatures. The assumption was that an energy barrier existed, preventing efficient collisions between HeH⁺ and hydrogen atoms. That would have made the ion less effective as a coolant than originally assumed.
Related Stories
- Researchers just made precise quantum vibrations that can detect individual molecules
- New bio-engineered molecule kills cancer cells and activates the immune system
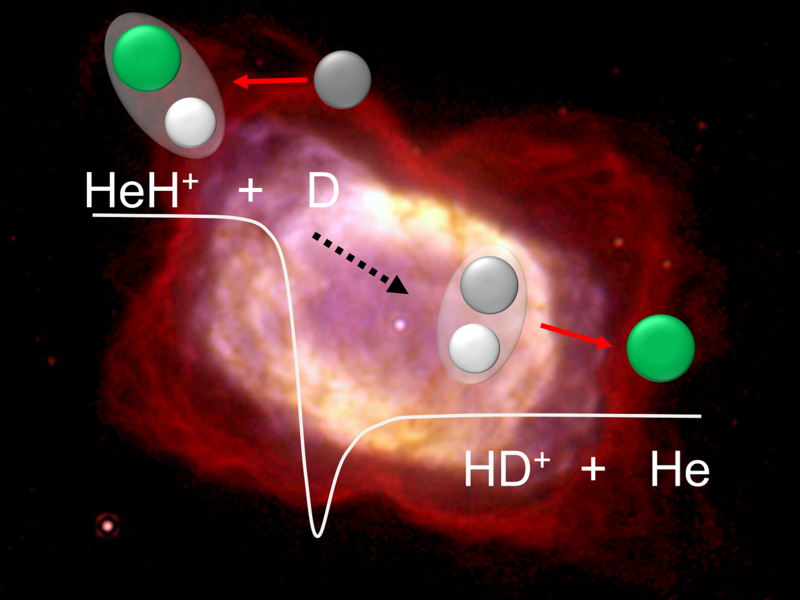
But new experiments suggest otherwise. Researchers at the Max Planck Institute for Nuclear Physics in Germany used a device known as the Cryogenic Storage Ring to store helium hydride ions at extremely cold temperatures, as low as minus 449 degrees Fahrenheit (minus 267 degrees Celsius). After holding the ions for up to a minute, the team directed beams of deuterium—heavy hydrogen atoms—toward them to observe what happened.
What they found was striking. The ions reacted quickly, without any sign of slowing down at low temperatures. “Previous theories predicted a significant decrease in the reaction probability at low temperatures, but we were unable to verify this in either the experiment or new theoretical calculations,” said Holger Kreckel, a study co-author at Max Planck.
The results confirm that the reaction is “barrierless,” meaning nothing blocks the ions from interacting efficiently. That makes helium hydride far more effective as a cosmic coolant than past models had indicated.
Rethinking early star formation
If helium hydride ions could stay reactive even in the cold, they likely played a much larger role in the chemistry of the early cosmos than once believed. These ions would have helped kick off the formation of molecular hydrogen and hydrogen deuteride, both essential coolants during the universe’s “dark ages,” the long stretch before stars first lit up.
“Reactions between the ions and other atoms appear to have been far more important for chemistry in the early universe than previously assumed,” Kreckel said.
This finding helps resolve a long-standing puzzle in cosmology. Previous computer models underestimated the rate at which these reactions occurred, leading to inconsistencies in simulations of how quickly the first stars could form. The new study shows those earlier models likely relied on flawed energy surfaces that created an artificial slowdown. With updated data, simulations of early star formation can now be made more accurate.
Cooling the path to light
To appreciate why this matters, imagine the universe during its first billion years. Without efficient coolants like HeH⁺, gas clouds would have struggled to reach the densities needed for fusion. That delay could have slowed the appearance of stars, galaxies, and eventually planets. The fact that helium hydride ions were busy cooling the cosmos means the road to light may have been smoother and faster than once thought.
Molecules like HeH⁺ may sound like obscure chemical footnotes, but they acted as cosmic facilitators. They helped transform the universe from a uniform, hot fog into a place with structure, light, and eventually life.
Practical Implications of the Research
By showing that helium hydride ions remain reactive even in frigid conditions, this study gives scientists a more accurate picture of how the first stars formed. Updated models of these reactions will allow researchers to better simulate the early universe and understand the timeline of cosmic evolution.
Beyond astrophysics, the work also demonstrates how advanced laboratory techniques can mimic conditions billions of years old, opening doors to more discoveries about the chemistry of space.
For humanity, this knowledge deepens our grasp of how the cosmos transformed into a place capable of hosting galaxies, stars, planets, and life itself.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.