Strange parasite kills human cells and wears their remains as disguise
Scientists uncover how Entamoeba histolytica evades immune attacks, using genetic tools like RNAi and CRISPR for future treatments.
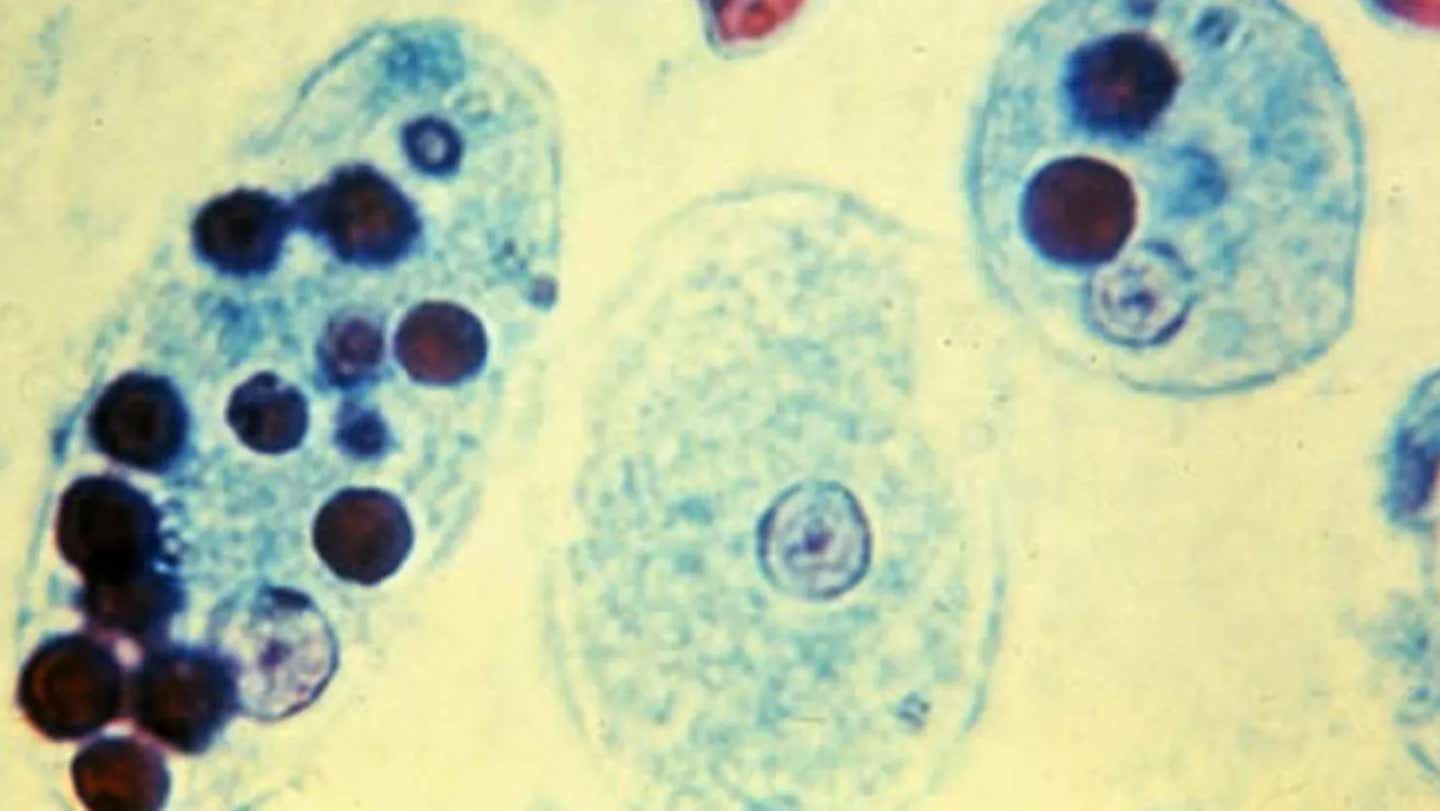
Entamoeba histolytica in the trophozoite stage. (CREDIT: CC BY-SA 4.0)
Entamoeba histolytica infects nearly 50 million people each year and kills around 70,000. It’s a shape-shifting parasite that invades the human gut, often causing mild symptoms like diarrhea. But in some cases, it becomes deadly. It can chew ulcers into the colon, liquefy parts of the liver, and even spread to the brain and lungs.
This single-celled killer is hard to study, even though it causes major illness and death, especially in developing countries. Scientists have long struggled to understand how it causes damage and evades the immune system. But thanks to years of careful work, they are finally beginning to build the tools needed to unlock its secrets.
A parasite that eats its victims alive
Once inside the body, Entamoeba histolytica heads straight for the colon. It usually spreads through food or water contaminated with feces. Poor sanitation makes the infection common in many parts of the world. In the U.S., it mostly appears in people who’ve recently traveled abroad or immigrated.
The species name “histolytica” means tissue-dissolving. And that’s exactly what it does. It doesn’t digest cells neatly. Instead, it tears off chunks of living tissue, leaving open wounds and liquefied tissue behind. These pockets of damaged tissue, called abscesses, are painful and sometimes fatal.
Back in 2011, Katherine Ralston began studying the parasite during her postdoctoral work at the University of Virginia. Most experts then thought the amoeba killed by injecting toxins. But when Ralston observed it under a microscope, she saw something else entirely.
The parasite was biting human cells, not poisoning them.
“You could see little parts of the human cell being broken off,” said Ralston, now an associate professor at UC Davis. Fluorescent dyes made the bitten cell fragments glow green under her microscope. These glowing pieces piled up inside the parasite, proving it had taken them in.
Related Stories
This process, called trogocytosis, was described in her 2014 paper in Nature. The discovery was a major breakthrough. “To devise new therapies or vaccines, you really need to know how E. histolytica damages tissue,” Ralston said.
How the parasite hides in plain sight
In 2022, Ralston and her team discovered another trick the parasite uses to survive. After biting human cells, it becomes resistant to the immune system. In a paper shared on bioRxiv in October 2024, Ralston and graduate students Maura Ruyechan and Wesley Huang explained how.
They found that the amoeba swipes proteins from the outer layer of human cells and puts them on its own surface. These stolen proteins, CD46 and CD55, stop the immune system’s “complement proteins” from attacking. In effect, the parasite kills human cells and then wears their protein coats as a disguise.
“It can kill anything you throw at it, any kind of human cell,” Ralston said. Even white blood cells meant to fight it can fall victim.
This type of stealth attack explains why the parasite can be so deadly. It not only attacks tissue directly, but also hides from the very system that should be stopping it.
A complex and stubborn genome
One reason why this parasite remained so mysterious for so long is that its genome is extremely complex. It was first sequenced in 2005 using a shotgun sequencing method. The strain used, HM-1:IMSS, was taken from a patient with dysentery and is still the most widely studied.
The genome is about 23.8 million base pairs in size, with nearly 10,000 predicted genes. Many of these genes—about 25%—have introns, and 6% have more than one intron. Roughly 75% of the genome is made up of adenine and thymine. It has between 31 to 35 linear chromosomes over 300,000 base pairs long, and about 200 circular DNA molecules that contain rRNA genes.
Despite being tetraploid—having four copies of each chromosome—its gene expression doesn’t follow the usual rules. More copies of a gene don’t necessarily mean more of its protein. That’s because the parasite uses RNA interference, or RNAi, to control gene expression. This acts like a volume knob that can turn genes up or down.
The genome is full of repeated sequences, making it difficult to assemble. The first published version had 888 contigs—far more than the actual number of chromosomes—indicating it was incomplete. Even so, scientists found signs of lateral gene transfer from bacteria and a massive expansion of certain gene families. These included genes for cysteine proteases, Rab and Arf proteins for vesicle transport, and regulators of the actin cytoskeleton.
The genome also lacks genes to make thymidylate, purines, pyrimidines, and fatty acids from scratch. It has no mitochondrial DNA, and the organelles known as mitosomes don’t contain DNA, which fits with its unusual biology.
Turning genetic tools into weapons
Progress has been slow, but it’s starting to pay off. In 2013, scientists showed that E. histolytica uses RNAi to manage gene expression. That gave researchers an idea: what if they could turn this natural process into a lab tool?
In 2021, Ralston and her team published a paper demonstrating a new RNAi library. This powerful tool lets scientists block each of the parasite’s 8,734 known genes one by one. By silencing individual genes, they can test which ones are essential for the parasite to infect cells, steal proteins, or avoid immune attacks.
In their latest paper, published in Trends in Parasitology, Ralston, Huang and Ruyechan explain how to use this tool to find the parasite’s weak spots. They suggest pairing RNAi with CRISPR/Cas9 gene editing. By doing this, they could label proteins with fluorescent tags to watch them in real-time or cut out small sections of DNA to see what’s critical.
“We now see a light at the end of the tunnel,” said Huang. “And we think this could be achievable.”
The idea is to build on each step. Discover which genes matter most. See how they help the parasite bite cells or dodge the immune system. Then find drugs that can block these steps.
How basic science paves the road to cures
The long journey to understand this parasite shows how basic research leads to real progress. Growing E. histolytica in the lab wasn’t easy. Sequencing its genome took years. Finding that it bites human cells and steals their proteins took more.
But now, armed with a working RNAi system and close to using CRISPR, researchers are positioned to discover the parasite’s most important genes and proteins. These discoveries may point the way to vaccines or new medicines that could save thousands of lives.
“Science is a process of building,” Ralston said. “You have to build one tool upon another, until you’re finally ready to discover new treatments.”
This work is already laying the foundation for that future. And for one of the world’s most overlooked parasites, that could mean the difference between deadly silence and finally being heard.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.



