Virus found in 94% of adults could be the cause of Lupus, study finds
Several hundred thousand Americans, and about 5 million people worldwide, develop systemic lupus erythematosus, better known as lupus.

 Edited By: Joshua Shavit
Edited By: Joshua Shavit
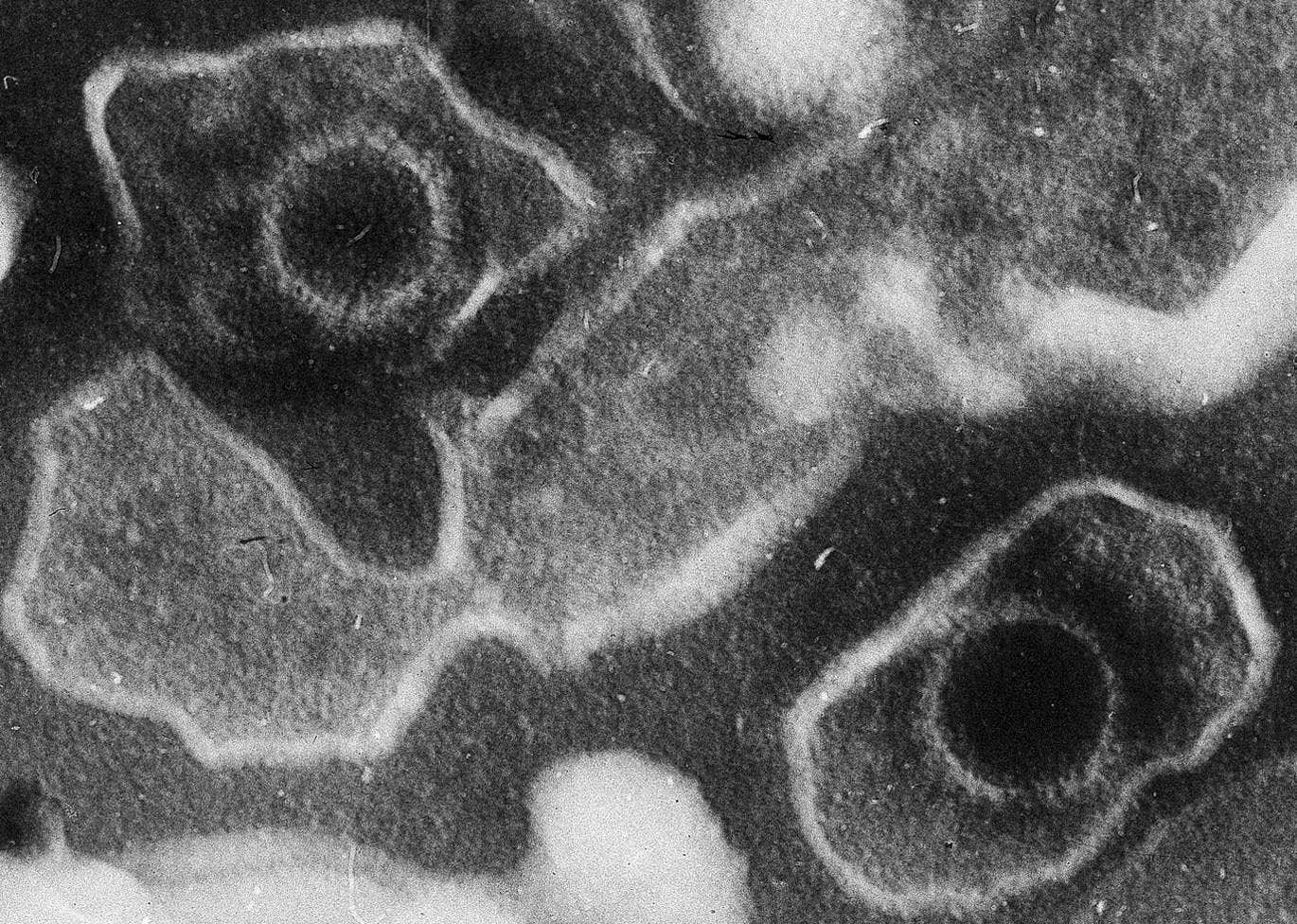
This electron microscopic image of two Epstein Barr Virus virions (viral particles) shows round capsids—protein-encased genetic material—loosely surrounded by the membrane envelope. (CREDIT: Wikimedia / CC BY-SA 4.0)
If you live in the United States, odds are high that a tiny passenger rides in your blood. By adulthood, more than 94 percent of people carry Epstein-Barr virus, or EBV, a germ that often spreads through shared drinks or a first kiss and can cause mononucleosis. For most, it quietly settles into the immune system and never makes much noise again.
For a much smaller group, though, life takes a very different turn. Several hundred thousand Americans, and about 5 million people worldwide, develop systemic lupus erythematosus, better known as lupus. In this chronic disease, the immune system attacks the nuclei inside your own cells and can damage skin, joints, kidneys, nerves and more. Most patients are women, and symptoms range from rashes and joint pain to kidney failure and heart problems.
For decades, scientists saw hints that EBV and lupus were connected. Almost everyone with lupus tests positive for EBV. People with lupus tend to have higher levels of antibodies against the virus, and EBV often reawakens around the time symptoms flare. What stayed unclear was how a nearly universal virus could help drive a rare and serious autoimmune disease.
A new study, led by Stanford Medicine investigators and their colleagues and published in Science Translational Medicine, offers a detailed answer. It suggests that in lupus, EBV does much more than tag along. It helps turn a small group of misdirected immune cells into ringleaders of a body-wide attack.
A common virus meets vulnerable immune cells
Lupus centers on B cells, immune cells that make antibodies and help alert the rest of your immune system to danger. Your body carries hundreds of billions of B cells. Together, they can recognize an estimated 10 billion to 100 billion different shapes on viruses, bacteria and other threats.
Because this diversity comes from an error-prone process, about 20 percent of your B cells are “autoreactive.” They can recognize parts of your own tissues, including DNA and proteins inside cell nuclei. Most of the time, these self-targeting cells stay lazy and quiet, and your body has checks that keep them from causing harm.
EBV complicates that balance. The virus belongs to the herpes family and can tuck its genetic material into B cells, where it often lies dormant for years. “Practically the only way to not get EBV is to live in a bubble,” said William Robinson, MD, PhD, a professor of immunology and rheumatology at Stanford and senior author of the study. “If you’ve lived a normal life,” he said, the odds are nearly 20 to 1 you are infected.
Earlier work showed that nearly everyone with lupus carries EBV, but so do most people without the disease. The question was why only a fraction of infected people develop autoimmunity.
Tracking infected B cells one at a time
Part of the challenge is numbers. In a typical healthy person, fewer than 1 in 10,000 B cells harbor EBV. Those infected cells are scattered, quiet and hard to spot with standard tools.
Robinson’s team built a more sensitive method they call EBV-seq. It is a version of single-cell RNA sequencing that adds 23 extra primers, short pieces of genetic material that latch onto 21 EBV genes. That tweak boosted viral gene detection about fourfold in test cell lines and allowed researchers to pick out infected cells that older methods missed.
The group then examined blood B cells from 11 people with lupus and 10 matched healthy volunteers. All had EBV, based on antibody tests. Using EBV-seq together with several other single-cell methods, the scientists profiled the types of B cells present and flagged any that carried viral genetic material.
The difference between groups was sharp. In people with lupus, they found on average about 25 EBV-infected B cells for every 10,000 B cells studied. That is roughly 1 in 400 cells, and some patients had more than 80 infected cells per 10,000. In healthy participants, the average was just 1 infected cell per 10,000, and some had none. Overall, lupus patients showed about 25 times more EBV-positive B cells than healthy controls, a highly significant jump.
When the team mapped those infected cells onto known B cell types, most of them clustered in a group called CD27+CD21 low memory B cells. These cells already have features linked to autoimmunity. Many are “antinuclear” B cells, which can recognize nuclear material. Smaller numbers of infected cells turned up in other memory B cells and in nondividing plasmablasts, the cells that can quickly release antibodies.
How EBV rewires B cells into “driver” cells
The researchers then asked a key question: What changes once EBV moves into these vulnerable B cells?
They looked at human gene activity inside infected and uninfected CD27+CD21 low memory B cells from people with lupus. EBV-positive cells switched on pathways tied to viral responses, antigen presentation, B cell activation and interferon signaling. They also showed higher levels of signaling molecules such as BTK, BLNK, JAK3 and PIK3R1, which make B cells more responsive to triggers.
Many of the most increased genes help B cells act as powerful antigen-presenting cells. Genes such as CD70, IFI30, TAP2 and PSMB6 boosted the cells’ ability to process pieces of nuclear material and display them on their surface for T cells to see. In people with lupus, EBV-infected B cells were better equipped to show both class I and class II antigens, which can activate a wide range of T cells.
The viral protein EBNA2 played a central role. EBNA2 acts as a transcription factor, a molecular switch that turns human genes on and off. By lining up their EBV-seq data with earlier studies of EBNA2 binding in B cell lines, the scientists saw that EBNA2 often sat near the start sites of genes that were more active in infected cells. At genes such as CD27, CD70, ZEB2 and TBX21, EBNA2 appeared to help open up nearby chromatin and recruit the cell’s own RNA polymerase, making gene transcription easier.
EBV-Positive B Cells and Immunity Suppression
At the same time, EBV-positive B cells raised levels of molecules such as CD73, CD39 and TGF-beta. Together, these create a small pocket of immune suppression around the infected cell, which can weaken antiviral T cells and allow infected B cells to survive longer.
The team also studied the antibodies made by these cells. They recreated 69 monoclonal antibodies from EBV-positive B cells in people with lupus. About 64 percent of those antibodies stained HEp-2 cells, a lab test used to detect antinuclear antibodies. Many bound classic lupus autoantigens, including double-stranded DNA, Smith antigen, Ro52, Ro60, histone H3 and PCNA. Some antibodies also recognized EBV’s EBNA1 protein, evidence of “molecular mimicry,” where one antibody hits both viral and self targets.
In comparison, none of the 10 antibodies from EBV-positive B cells in healthy volunteers and none of the 15 from people with multiple sclerosis reacted with lupus-related autoantigens.
When researchers grouped B cells into clonal families based on shared receptor sequences, they often found a single EBV-infected cell surrounded by many uninfected relatives that had accumulated more mutations and stronger autoreactivity. That pattern supports the idea that infected B cells act as “driver” cells. They present nuclear material, draw in T helper cells, and help both related and unrelated antinuclear B cells grow and sharpen their aim.
A new way to see lupus
To test that idea, the team created EBV-transformed lymphoblastoid cell lines from people with lupus who had anti-DNA antibodies. They loaded those cells with chromatin, the mix of DNA and proteins that makes up nuclei, and grew them with each person’s own T and B cells.
After 10 days, single-cell analysis showed that a specific group of T cells called peripheral helper T cells had expanded. So had uninfected DN2 B cells and plasmablasts, many of which produced antinuclear antibodies. Culture fluids from these matched lupus cell mixes contained higher levels of ANAs than mismatched or healthy samples.
Taken together, the findings offer a step-by-step explanation. In people at risk for lupus, EBV infects B cells that already recognize nuclear material. Viral proteins such as EBNA2 rewire those cells into potent antigen presenters and local immune shapers. These “driver” cells call in T helper cells, which then support large groups of autoreactive B cells, most of them uninfected. Over time, those partner cells mature and produce high-affinity antinuclear antibodies that damage tissues across the body.
“This is the single most impactful finding to emerge from my lab in my entire career,” Robinson said. “We think it applies to 100% of lupus cases.”
The work also helps answer a long-standing puzzle: If about 95 percent of people carry EBV, why do so few develop lupus? The study suggests that infection alone is not enough. A person likely needs the right mix of genetic risk, autoreactive B cells that escape normal checkpoints, and possibly specific strains of EBV that are more likely to create driver cells.
Research findings are available online in the journal Science Translational Medicine.
Related Stories
- Scientists have finally discovered the cause of lupus
- Lifechanging lupus discovery could result in a cure - helping 1.5 million people in the U.S
- Nightmares could be an early warning sign of lupus and other autoimmune diseases
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joseph Shavit
Writer, Editor-At-Large and Publisher
Joseph Shavit, based in Los Angeles, is a seasoned science journalist, editor and co-founder of The Brighter Side of News, where he transforms complex discoveries into clear, engaging stories for general readers. With vast experience at major media groups like Times Mirror and Tribune, he writes with both authority and curiosity. His writing focuses on space science, planetary science, quantum mechanics, geology. Known for linking breakthroughs to real-world markets, he highlights how research transitions into products and industries that shape daily life.



