Weight-loss drug Tirzepatide significantly slows breast cancer growth, study finds
New study shows weight-loss drug tirzepatide slows breast cancer growth in obese mice, offering hope for dual-action treatment.
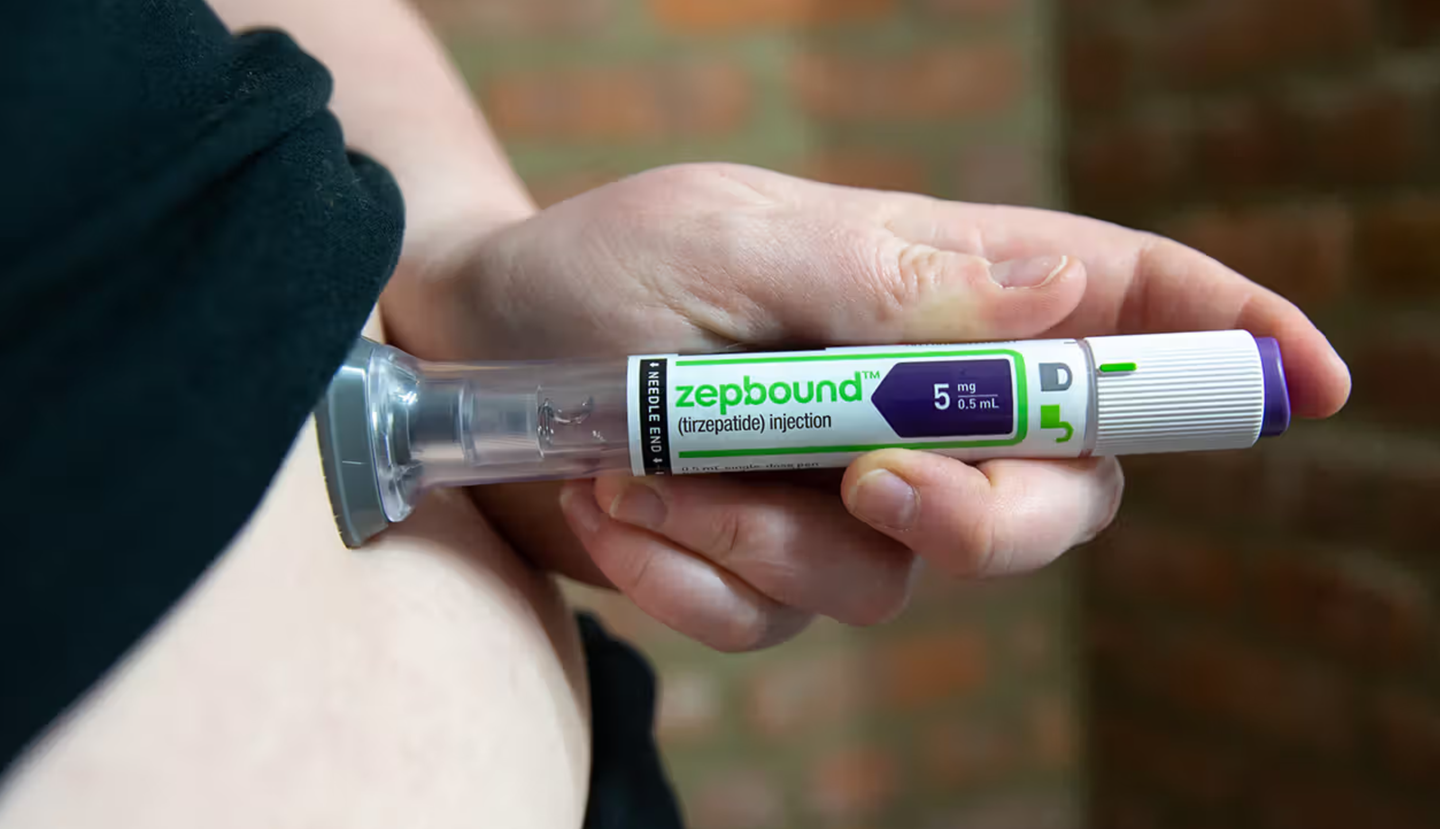
A new study reveals that tirzepatide, a popular anti-obesity drug, may also slow breast cancer growth by reducing body fat. (CREDIT: CC BY-SA 4.0)
Key Takeaways
- The weight-loss drug tirzepatide reduced both body fat and tumor growth in obese mice with breast cancer.
- Tumor size closely matched body weight and fat levels, suggesting fat reduction may slow cancer growth.
- Ongoing research aims to determine if tirzepatide’s anti-cancer effects go beyond just weight loss.
- The future looks bright for tirzepatide and other GLP-1 agonists based on recent clinical studies and regulatory developments.
In a promising new study presented at the Endocrine Society’s ENDO 2025 meeting in San Francisco, researchers reported that the drug tirzepatide, commonly used for treating obesity and type 2 diabetes, significantly slowed breast cancer growth in obese mice. These findings could pave the way for new treatments that target both obesity and its cancer-related consequences.
Tirzepatide, sold under the brand names Mounjaro for diabetes and Zepbound for obesity, works by stimulating two key hormone receptors, GLP-1 and GIP. These hormones help control blood sugar and reduce appetite. It has already shown remarkable success in helping people lose significant weight, but now researchers are exploring whether it can do more—such as reduce cancer risk in patients living with obesity.
Targeting a Major Risk Factor
Obesity is known to increase the risk of developing many chronic diseases, including breast cancer. According to Amanda Kucinskas, a Ph.D. candidate at the University of Michigan, “Obesity is a significant risk factor for breast cancer, and while it is very preliminary data, our studies in mice suggest that these new anti-obesity drugs may be a way to reduce obesity-associated breast cancer risk or improve outcomes.” Kucinskas works in the labs of Drs. Erin Giles and Kanakadurga Singer, both of whom focus on the links between metabolism and cancer.
Weight loss has been shown to improve cancer outcomes in people with obesity. However, many struggle to lose weight through diet and exercise alone. With new drugs like tirzepatide, researchers are beginning to ask whether addressing obesity pharmacologically could also reduce cancer growth.
Related Stories
- GLP-1 diabetes drugs like Ozempic found effective against migraines
- First head-to-head trial compares tirzepatide and semaglutide - Results are in!
- Common diabetes drugs may protect against Alzheimer’s disease
A Mouse Model with Human Implications
The study followed 16 mice bred to develop obesity. To simulate human obesity, the mice were fed a high-fat diet and kept in a warm environment to suppress calorie burning. At 32 weeks of age—roughly middle age for a mouse—the obese animals were divided into two groups. One group received tirzepatide injections every other day for 16 weeks. The other received a placebo. Scientists measured the mice’s weight and tumor growth twice a week throughout the study.
Mice that received tirzepatide lost about 20% of their body weight. This degree of weight loss closely mirrors what human patients experience on the medication. The fat loss occurred mainly in adipose tissue, the body's fat-storing cells. Compared to the control group, the tirzepatide-treated mice showed much smaller fat deposits.
Shrinking Tumors Along with Waistlines
The weight loss wasn’t the only good news. Mice treated with tirzepatide also had significantly smaller tumors. Researchers found a clear link between lower body weight and smaller tumor size. Total fat mass and liver fat were also strongly linked to how large the tumors became. These findings suggest that reducing fat in the body might play a direct role in slowing cancer growth.
“This is an early but exciting result,” said Kucinskas. “It suggests that this new anti-obesity drug may also have a beneficial impact on breast cancer outcomes.”
Although this research was conducted in mice, it points to important questions about how obesity, metabolism, and cancer growth are connected. Human studies will be needed to confirm whether the benefits observed in mice apply to people.
Next Steps in the Research
To learn more, Kucinskas and her team are continuing their work in collaboration with Dr. Steve Hursting’s lab at the University of North Carolina at Chapel Hill. This next phase aims to figure out whether tirzepatide's effects on tumors come only from weight loss, or if the drug also has cancer-fighting effects of its own, independent of fat loss.
By teasing apart these effects, scientists hope to better understand how medications like tirzepatide can be used to treat not just obesity, but also its complications—including breast cancer.
If future studies confirm these findings in humans, it could mean a major shift in how doctors treat breast cancer in patients who are overweight or obese. Drugs like tirzepatide might one day serve a dual role—helping patients manage their weight and protect against cancer progression.
Other Health Benefits of GLP-1 Drugs
Migraine Treatment:
A small pilot study led by neurologist Dr. Simone Braca at the University of Naples explored whether GLP‑1 receptor agonists—diabetes/weight‑loss medications like liraglutide (similar to Ozempic and Wegovy)—could help chronic migraine sufferers.
Over 12 weeks, obese participants with frequent migraines (around 20 headache days per month) received daily liraglutide. By the study’s end, average monthly migraine days dropped sharply to about nine, and migraine-related disability scores halved—even though weight loss in participants was minimal.
Researchers propose the relief lies not in weight reduction, but in GLP‑1s’ ability to decrease intracranial pressure. Evidence from animal and early human studies suggests these drugs act on the brain’s choroid plexus to slow cerebrospinal fluid production and lower levels of migraines‑triggering CGRP. This mechanism appears distinct from metabolic effects and addresses pressure-related migraine pathways.
Though results are promising—about half of participants experienced at least a 50% reduction in migraine days, and most saw improvement within two weeks—the study had no placebo control and a small sample size. Mild side effects like nausea or constipation occurred in around 38% of cases, but no one dropped out. The team plans to conduct larger, randomized, controlled trials to confirm these findings and explore whether other GLP‑1 drugs offer similar benefits with fewer gastrointestinal symptoms
Alzheimer's disease:
A major observational study led by researchers at the University of Florida examined Medicare data for over 90,000 older adults with type 2 diabetes. It compared patients initiating treatment with two classes of diabetes medications—GLP‑1 receptor agonists (GLP‑1RAs) and SGLT2 inhibitors (SGLT2is)—to those using other glucose-lowering drugs.
The findings revealed a significantly lower incidence of Alzheimer’s disease and related dementias among users of GLP‑1RAs (a 33% risk reduction, hazard ratio 0.67) and SGLT2is (43% reduction, hazard ratio 0.57), with no statistical difference between the two classes.
Beyond managing blood sugar, GLP‑1RAs and SGLT2is offer additional biological effects that might protect the brain. GLP‑1RAs mimic a hormone that enhances insulin release and digestion, while SGLT2is promote glucose excretion via urine. Beyond these roles, both drug classes appear to reduce inflammation, improve circulation, and even act on neuronal pathways—effects that collectively may help combat Alzheimer's pathology.
The research team, including UF professor Serena Guo, suggests these medications could be repurposed for brain health and possibly even used preventively in individuals without diabetes in the future. However, as the study is observational, it cannot definitively establish causation—clinical trials will be needed to confirm whether these drugs actively protect against cognitive decline .
Heart Health:
Recent analyses highlighted that GLP‑1 receptor agonists—like tirzepatide and semaglutide—deliver cardiovascular benefits that extend well beyond simple weight loss. These medications have been shown to improve heart function, shrink cardiac adipose tissue, and reduce left ventricular mass in patients with obesity-related heart conditions. Experts like Dr. Harlan M. Krumholz, from Yale University, underscore that such findings deepen our understanding of how targeted obesity treatments can positively impact heart health.
In the SUMMIT trial, tirzepatide—which acts on both GLP‑1 and GIP receptors—demonstrated significant cardiac remodeling effects: users experienced an average reduction of 11 grams in left ventricular mass and a 45‑milliliter decrease in surrounding fat.
This structural change is thought to underlie the drug’s capacity to lower heart failure events in patients with preserved ejection fraction. In parallel, data from the SELECT trial involving semaglutide revealed a notable decrease in recurrent cardiovascular risks among overweight or obese individuals who had undergone bypass surgery, signaling its promise as a secondary prevention therapy.
Beyond pharmaceuticals, lifestyle interventions are also making measurable impacts. The LookAHEAD trial, which centered on weight reduction for individuals with type 2 diabetes, found that losing weight improved levels of biomarkers associated with heart failure and atherosclerosis—specifically NT‑proBNP and hs‑cTnT.
Altogether, these findings suggest a powerful synergy: GLP‑1–based medication paired with structured lifestyle changes may offer a dual approach to managing obesity while enhancing cardiovascular health—potentially reshaping treatment strategies for chronic heart and metabolic conditions.
The prognosis for tirzepatide and other GLP-1 receptor agonists looks exceptionally promising based on recent clinical studies and regulatory developments.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.