Breakthrough new therapy regrows damaged cartilage in hours
Scientists have long dreamed of ways to regrow cartilage instead of replacing joints, but adult bodies lack the natural ability to rebuild this tissue.

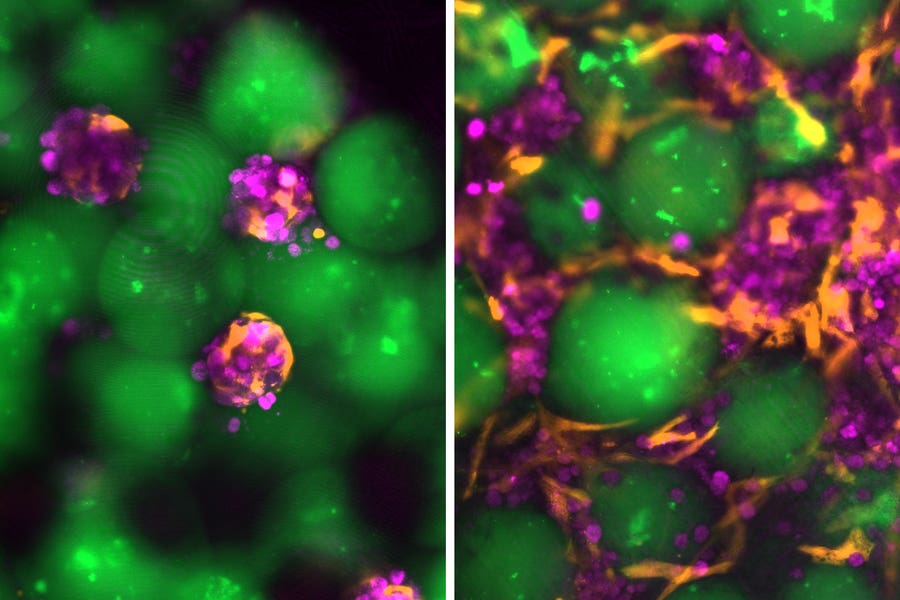
A new therapy using “dancing molecules” shows promise for regrowing cartilage, offering fresh hope for osteoarthritis patients worldwide. (CREDIT: Shutterstock)
Cartilage cushions your joints, letting them move smoothly. But with time, injury, or disease, that cushion can wear away. When it does, the result is often osteoarthritis—a painful condition that affects hundreds of millions of people worldwide. In its advanced stages, the cartilage disappears completely, leaving bones to rub against each other. For many, the only way forward is joint replacement surgery, which is expensive, invasive, and slow to recover from.
Scientists have long dreamed of ways to regrow cartilage instead of replacing joints, but adult bodies lack the natural ability to rebuild this tissue. That limitation may now be changing. Researchers at Northwestern University have developed a therapy made of “dancing molecules” that could coax cartilage cells into repairing themselves. What started as an idea for spinal cord healing has now opened the door to regenerating one of the body’s most stubborn tissues.
From Spinal Cords to Joints
The new work builds on discoveries first reported in 2021, when Samuel I. Stupp and his team revealed that specially designed molecules could spark spinal cord repair in mice. The surprising part wasn’t only the recovery itself, but how it happened. The molecules weren’t static. They were in constant motion, swirling and shifting, almost as if they were dancing. That movement turned out to be key.
“When we first observed therapeutic effects of dancing molecules, we did not see any reason why it should only apply to the spinal cord,” Stupp explained. “Now, we observe the effects in two cell types that are completely disconnected from one another — cartilage cells in our joints and neurons in our brain and spinal cord. This makes me more confident that we might have discovered a universal phenomenon. It could apply to many other tissues.”
The results were published in the Journal of the American Chemical Society. They suggest that motion itself might serve as a kind of medicine. By moving more, the molecules could interact with receptors on cells more efficiently, setting off repair pathways inside the body.
How Dancing Molecules Work
Cells communicate with their environment through proteins called receptors, which sit on the cell surface. These receptors aren’t stationary; they shift constantly, scanning their surroundings for signals. Stupp’s team reasoned that if their synthetic molecules could move in a similar way, they’d be better at connecting with these receptors.
Related Stories
- Breakthrough artificial knee cartilage is more durable than natural tissue
- New light-activated injectable gel heals bone faster and safer
The molecules, called peptide amphiphiles, self-assemble into fibers that resemble the body’s extracellular matrix—the supportive network that surrounds cells. The researchers built a special circular peptide that mimics transforming growth factor beta-1 (TGF-β1), a protein critical for cartilage formation. Instead of injecting fragile TGF-β1 proteins, which break down quickly and can cause harmful side effects, they placed this stable mimic on the surfaces of their nanofibers.
To test the importance of motion, the scientists made two versions of these nanofibers. One allowed more flexibility in the peptide signals, while the other held them more rigidly. Both versions could activate receptors, but the more mobile version worked far better.
“After three days, the human cells exposed to the long assemblies of more mobile molecules produced greater amounts of the protein components necessary for cartilage regeneration,” Stupp said. Remarkably, in producing one essential cartilage component, collagen II, the dancing molecules outperformed even natural TGF-β1.
Rapid Cell Response
The therapy acted with unexpected speed. Within just four hours, treated cartilage cells began expressing key genes linked to tissue repair. By the third day, they were producing major proteins needed to build new cartilage, including collagen II and aggrecan. This swift response surprised even the research team.
Imaging also showed that treated cells kept a healthy, rounded shape, unlike untreated cells, which often take on a stressed, degenerative look. When the nanofibers were assembled into hydrogels, creating a 3D environment closer to real tissue, they continued to support healthy cartilage cells. The gel kept the cells dispersed, alive, and active in producing matrix proteins.
The promise of this discovery doesn’t stop at joints. Stupp and his group are already testing the molecules in other tissues. Early results suggest they might also help repair bone. Experiments in human organoids—tiny lab-grown tissues—are underway to speed the development of new therapies. The team is also building the scientific foundation needed for clinical trials aimed at spinal cord injuries.
“We are beginning to see the tremendous breadth of conditions that this fundamental discovery on ‘dancing molecules’ could apply to,” Stupp said. “Controlling supramolecular motion through chemical design appears to be a powerful tool to increase efficacy for a range of regenerative therapies.”
A New Direction for Osteoarthritis Treatment
Osteoarthritis affected nearly 530 million people worldwide in 2019, and cases continue to rise as populations age. Most treatments today focus on reducing pain or slowing down joint damage. None restore cartilage once it’s gone. Stupp’s approach could change that. Instead of masking symptoms or buying time before surgery, these molecules aim to rebuild the lost tissue itself.
For patients, the difference could be life-changing. A therapy based on dancing molecules might offer a way to heal joints without resorting to replacement surgery. It could also extend mobility and independence for millions of older adults.
If these findings translate successfully to humans, the impact would be enormous. For people with osteoarthritis, the therapy could mean fewer joint replacements and a higher quality of life. Athletes and those with cartilage injuries might recover faster and more fully. Beyond cartilage, the approach could spark regenerative treatments for bone, spinal cord damage, and possibly even brain injuries.
By designing molecules that move in sync with cell receptors, scientists may have uncovered a universal way to trigger healing in tissues that rarely repair themselves. That insight could guide the next generation of biomaterials, leading to therapies that are not only more effective but also safer and more durable than current options.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.