3D-printed heart patch made of living cells regrows damaged muscle
New biodegradable 3D-printed heart patch merges with heart tissue, repairing damage and vanishing after healing.
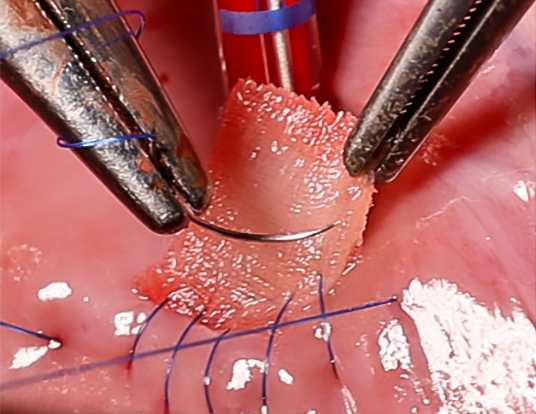
The support scaffold is implanted into the heart defect and covered with the mesh. (CREDIT: Soft Robotics Lab / ETH Zurich)
A heart attack cuts off blood flow and oxygen to heart tissue. This can damage muscle and, in severe cases, cause the heart wall to rupture. Surgeons often repair these defects with patches made from bovine pericardium, the thin membrane surrounding a cow’s heart. These patches are valued for stability, permeability, and ease of surgical use.
Yet these materials are far from perfect. They remain in the body as foreign objects, never breaking down or merging with heart muscle. Over time, they can trigger inflammation, calcification, or blood clots. “Traditional heart patches do not integrate into the heart tissue and remain permanently in the body,” says Lewis Jones, lead author of a new study. “We wanted to solve this problem with our patch, which integrates into the existing heart tissue.”
Building a patch that heals, not just seals
A team from ETH Zurich and the University Hospital of Zurich, led by Professors Robert Katzschmann and Omer Dzemali, has developed a patch designed to do more than close a wound. Featured in Advanced Materials, the reinforced cardiac patch—called the RCPatch—aims to restore heart function by merging with natural tissue and gradually disappearing as the heart heals.
“Our goal was to develop a patch that not only closes a defect but also helps to repair it completely,” says Katzschmann. Unlike current bovine pericardial patches, this design offers biological activity. It is built to degrade once it has done its job, leaving no foreign material behind.
The RCPatch combines three main elements: a fine mesh to seal the defect, a 3D-printed scaffold for stability, and a hydrogel infused with living heart muscle cells. The scaffold, made from a biodegradable polymer called polycaprolactone, has a lattice pattern created through high-precision volumetric 3D printing. This structure gives it strength while allowing it to hold the hydrogel.
Jones explains, “The scaffold is stable enough and can be filled with a hydrogel containing living cells.” A thin, suturable mesh is added, also enriched with the hydrogel. This allows the patch to anchor securely to the heart wall and encourages integration with existing muscle tissue. Over time, the scaffold breaks down as the cells merge with the myocardium, leaving only repaired, living tissue.
Related Stories
- New light-activated heart tissue revolutionizes heart repair
- 500 years later, scientists solve Leonardo da Vinci’s heart mystery
Engineering for strength and biology
Designing an implant for the heart’s high-pressure environment is a balancing act. Soft materials like hydrogels are biologically friendly but too fragile to survive heart contractions and surgical handling. Stiffer synthetic materials offer strength but do not support cellular growth. The RCPatch bridges this gap by embedding the hydrogel within a mechanically tuned, biodegradable scaffold.
The research team also used a process called melt-electrowriting to produce the mesh, which was then soaked in fibrin hydrogel to make it less permeable. This reduces the risk of blood leakage while keeping the patch light and flexible enough to suture.
The metamaterial design is anisotropic, meaning its stiffness can vary in different directions—an important trait for matching the heart’s complex motion. Computational models helped optimize the lattice geometry so that the patch could withstand pressure while still moving with the heart’s natural rhythm.
Testing under real-world pressures
Initial trials on animal hearts showed the patch could be successfully implanted and hold up under normal cardiac pressures. In one study, an artificial defect in the left ventricle of pigs was sealed using the RCPatch.
The patch maintained its integrity even under blood pressures of 81 mmHg before surgery and 66 mmHg after implantation, while preventing bleeding and restoring stable heart function.
These results suggest the patch could work in human hearts as well. In future studies, the team plans to test the patch over longer periods to track how well it integrates, degrades, and supports regeneration.
Addressing long-standing challenges
Heart disease remains the leading cause of death worldwide. After a myocardial infarction, damaged areas often thin and lose strength, putting extra strain on healthy tissue. In worst cases, this leads to rupture, which requires rapid surgery.
Current patches, whether made from animal tissue or synthetic fibers like polytetrafluoroethylene or polyester, provide stability but no regeneration. They stay in the heart for life, which can cause problems, especially in younger patients whose hearts are still growing.
The ideal patch would be easy for surgeons to handle, strong enough to hold during healing, and biodegradable once new tissue forms. It would integrate fully with the heart, avoiding immune rejection or long-term complications.
Tissue-engineered heart patches—known as engineered heart tissues—have shown promise in animal models by contracting, conducting electrical signals, and developing blood vessels. But most of these have been designed for the outside of the heart, not for high-pressure intraventricular repairs.
The RCPatch represents a leap forward by combining the mechanical strength needed for internal placement with the living-cell structure required for regeneration. Its multi-material build, optimized geometry, and degradable composition make it a candidate for both adult and pediatric cases.
From lab to operating room
While this technology is still in the preclinical stage, the Zurich team’s work lays the foundation for a new class of surgical implants. If future trials confirm safety and long-term stability, the RCPatch could replace current inert materials in many cardiac surgeries. The ability to not only patch but also heal could shift how doctors think about post-heart-attack repairs.
Katzschmann believes the vision is clear: “In the long term, we want to see patches that don’t just close a defect but help the heart regenerate. That’s the real goal.”
For patients, this could mean fewer complications, faster recovery, and a patch that becomes part of their own heart—rather than a foreign object that must be lived with forever.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.



