A break-through in treatment of Cystic Fibrosis
Cystic fibrosis is characterized by the build-up of thick, sticky mucus in the lungs which renders breathing increasingly difficult.
[August 31, 2021: CHARITÉ - UNIVERSITÄTSMEDIZIN BERLIN]
Cystic fibrosis is characterized by the build-up of thick, sticky mucus in the lungs which renders breathing increasingly difficult. An increase in the amount of salt found in sweat is another key symptom, and one which makes diagnosis easy. The disease, which affects approximately one in 2,500 to 3,000 babies and usually manifests in early childhood, is the most common fatal genetic disease. Cystic fibrosis is caused by mutations in the CFTR gene, which is responsible for the building and normal functioning of a chloride channel and therefore essential for the transport of salt and water across epithelial surfaces of the airways and the skin. Researchers have so far identified more than 2,000 genetic defects which when affecting both copies of the gene can impair normal CFTR chloride channel functioning in a variety of ways.
For a long time, disease management relied solely on symptom-based treatments. A breakthrough finally occurred a few years ago, during the development of a triple combination therapy which uses ‘CFTR modulators’, compounds capable of targeting the disease’s underlying molecular defects. This treatment had not previously been available to patients with rarer forms of cystic fibrosis which are caused by very specific genetic defects. Now, however, this triple combination therapy has been shown to be highly effective even in patients whose disease is caused by these rare mutations. “This means that in the future nine in ten cystic fibrosis patients in Germany will be able to receive an effective treatment which targets the underlying molecular defects responsible for their disease,” explains co-first author Prof. Dr. Marcus A. Mall, Head of Charité’s Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine and Charité’s Cystic Fibrosis Center. He adds: “This represents a crucial step on the path to finding an effective treatment for all people with cystic fibrosis.”
The most common defect, known as the F508del mutation, affects approximately 90 percent of patients with cystic fibrosis. It causes a folding defect in the CFTR channel’s structure which renders the protein defective and stops it from being integrated into cell membranes. This folding defect (F) can be corrected with drugs known as ‘CFTR correctors’ (such as elexacaftor and tezacaftor), which bind to different sites on the CFTR protein and must be used in combination to produce effective improvements in channel folding. A spectrum of rare mutations of the gene can also result in: ‘minimal function mutations’ (MF), where the CFTR protein cannot be pharmacologically activated; ‘gating mutations’ (G), where the channel remains locked; and ‘residual function mutations’ (RF), where the channel shows impaired function. Drugs known as ‘CFTR potentiators’ can be used to improve the function of these mutated channels in the cell membrane. The new triple therapy, for instance, combines elexacaftor, tezacaftor and ivacaftor in order to achieve maximum therapeutic effect, improving both defective channel folding (caused by the F508del mutation) and the activity and functioning of channels with rare defects which have been integrated into cell membranes.
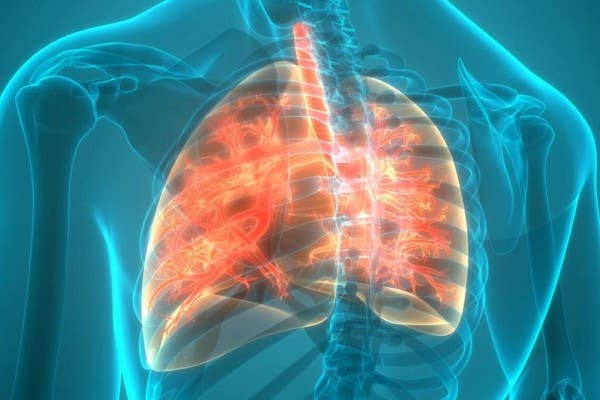
In cystic fibrosis, the airways fill with thick, sticky mucus, making it difficult to breathe. The thick mucus is also an ideal breeding ground for bacteria and fungi. (CREDIT: Mayo Foundation for Medical Eductaion and Research)
“The effect of this type of triple combination therapy on patients with an F508del mutation in addition to either a rare gating mutation (F/G) or residual function mutation (F/RF) had not previously been established. While the individual mutations occur only rarely, collectively they do affect approximately 15 percent of patients with cystic fibrosis,” says the Einstein Professorship holder who also directs cystic fibrosis research activities at the German Center for Lung Research (DZL). According to previous phase III trials (also conducted with Prof. Mall’s involvement), triple combination therapy produced significant increases in CFTR function and improvements in lung function and quality of life in 75 percent of patients with cystic fibrosis with either two copies of the F508del mutation (F/F) or one copy of the F508del mutation and a minimal function mutation (F/MF). These findings prompted the Europe-wide approval of this triple combination therapy for use in patients aged 12 and older presenting with these genetic mutations.
As part of their most recent randomized, controlled phase III trial, the researchers studied a total of 258 patients with either F/G or F/FR mutations – a huge number given the rare disease status of cystic fibrosis. Participants received either triple combination therapy or standard therapy (ivacaftor, either as monotherapy or in combination with tezacaftor). During an eight-week follow-up period, researchers studied changes in lung function and levels of chloride (salt) in sweat as biomarkers for CFTR function. Researchers also used a disease-specific questionnaire to estimate health-related quality of life. “When compared to standard therapy, triple combination therapy resulted in significant improvements in CFTR function, lung function and quality of life. “In keeping with results from previous studies, triple combination therapy was shown to be safe and well-tolerated in this particular patient group,” emphasizes Prof. Mall. He adds: “This drug-based approach, targeting changes in CFTR chloride channels caused by mutations on both gene copies, offers an even more effective way to treat this disease.”
Emphasizing that authorization for all age groups (ideally from infancy) will make effective treatment available to approximately 90 percent of patents, Prof. Mall explains: “Triple therapy holds enormous potential, particularly when used early on against the disease-causing molecular defects to prevent irreversible and previously life-limiting lung damage. Eventually, this will turn a previously fatal genetic disease into a treatable one.” Prof. Mall co-leads the global program coordinating the clinical development of triple combination therapy for use in children with cystic fibrosis. The clinical development of an early treatment option is therefore one focus of his team’s current research endeavors. The first randomized, controlled trial evaluating its safety and efficacy in preschool-age children with a F508del mutation in combination with a minimal function mutation (F/MF) is already underway.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Tags: #New_Discoveries, #Medical_News, #Cystic_Fibrosis, #Treatment, #Respiration, #Genetics, #The_Brighter_Side_of_News