First human trial of a CRISPR-based treatment significantly lowered cholesterol and triglycerides
A one time CRISPR infusion lowered cholesterol and triglycerides in an early trial, offering hope for people with hard to treat lipid disorders.

 Edited By: Joshua Shavit
Edited By: Joshua Shavit
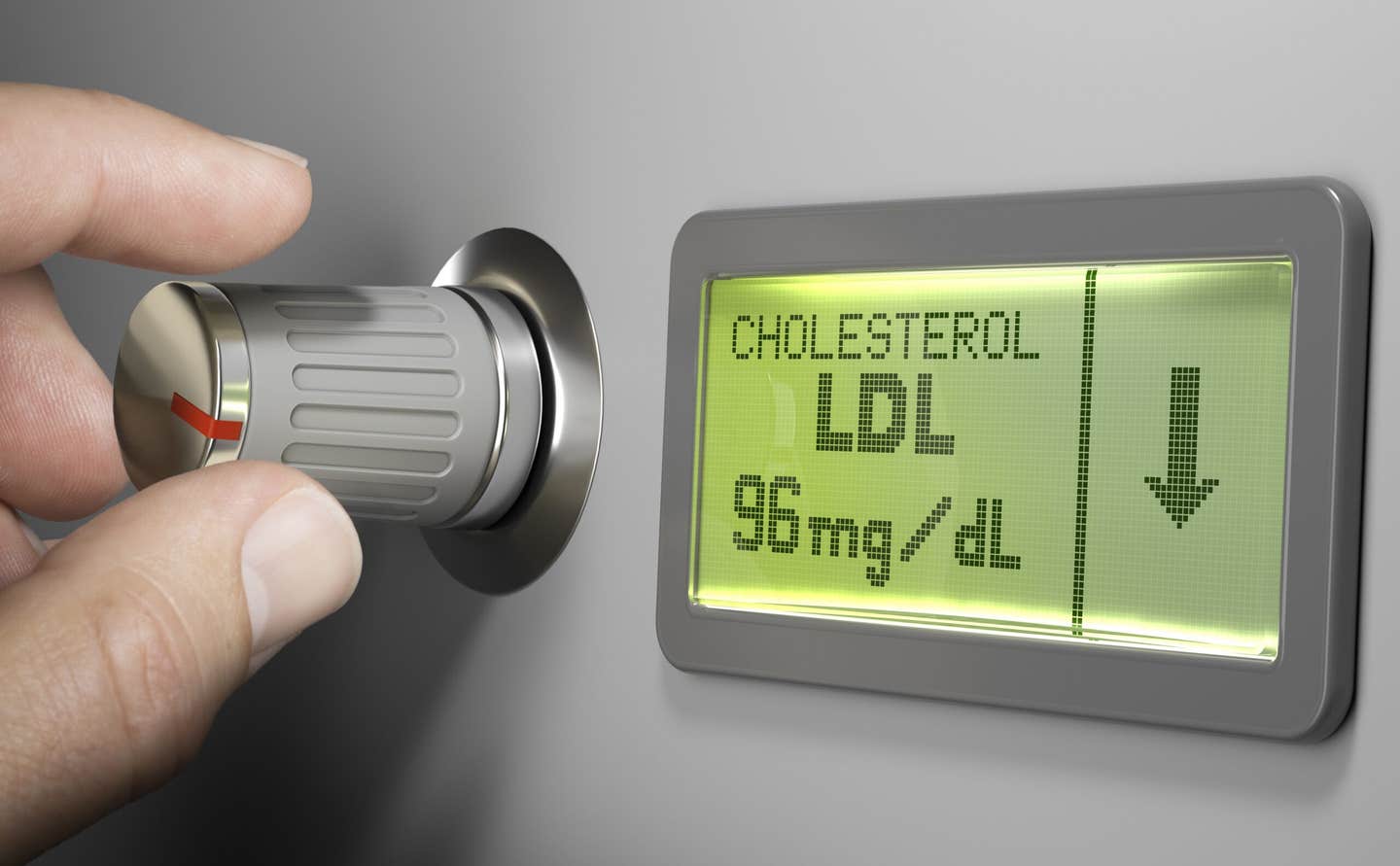
A first in human trial of a CRISPR based treatment has produced strong early drops in LDL cholesterol and triglycerides. (CREDIT: Shutterstock)
A quiet hope has started to grow among people living with stubborn cholesterol problems as researchers report early success with a new kind of treatment that works with only one infusion. The experimental therapy, called CTX310, uses CRISPR gene editing to switch off a liver gene that pushes cholesterol and triglycerides to harmful levels.
Early findings from a first-in-human Phase 1 trial suggest that one treatment may keep both blood fats low for months, and possibly longer, which has drawn strong interest in the cardiology community.
A One Time Infusion Designed to Tackle a Lifelong Risk
The treatment delivers CRISPR-Cas9 through tiny fat based particles that guide the editing tools into the liver. Once there, the treatment turns off a gene known as ANGPTL3. People born with a natural loss of this gene tend to have low LDL cholesterol and low triglycerides for life, without clear downsides. That natural clue encouraged researchers to explore whether gene editing could create the same lifelong change for people who have spent years fighting high lipid levels.
In the trial, researchers saw LDL cholesterol and triglycerides begin to fall within two weeks. Those levels stayed low through at least 60 days, which was the longest follow up included in the early data. The reduction was stronger than the team expected. A drop of 30 to 40 percent would have been enough to call the approach successful, yet the highest dose brought reductions of about 50 percent or more for both LDL cholesterol and triglycerides.
Luke J. Laffin, M.D., a preventive cardiologist at the Cleveland Clinic and lead author of the study, said the scale of the drop surprised the team. “This is really unprecedented. A single treatment that simultaneously lowered LDL cholesterol and triglycerides,” he said. He added that if the results continue to hold in larger groups, this approach could reshape care for people with complex lipid disorders and cut their lifetime risk of heart attacks and strokes.
Why Cholesterol Control Is So Difficult for Many People
High LDL cholesterol and high triglycerides raise the risk of clogged arteries. In the United States, millions of adults live with levels high enough to put them at risk. Statins, injections and lifestyle changes help many, but not everyone responds well. Some cannot tolerate high doses. Others find it hard to stay on treatment. Steven E. Nissen, M.D., chief academic officer at the Cleveland Clinic Heart, Vascular and Thoracic Institute and co author of the study, noted that many people stop taking cholesterol medicines within a year. He said the idea of a one time treatment with lasting effects could be a major step forward.
The trial enrolled 15 adults across six sites in Australia, New Zealand and the United Kingdom. Their ages ranged from early 30s to the mid 70s. Most had inherited lipid disorders, and all had elevated cholesterol or triglycerides despite taking the strongest treatment they could tolerate. One participant had homozygous familial hypercholesterolemia, a rare inherited condition that causes extremely high cholesterol starting in childhood. Others had severe triglyceride elevations or mixed lipid disorders that are notoriously hard to manage.
Participants received one infusion of CTX310 at doses between 0.1 and 0.8 mg per kilogram. Everyone was pre treated with steroids and antihistamines to reduce the chance of an infusion reaction. Three participants had brief issues like back pain or nausea that resolved with medication. One person who already had elevated liver enzymes saw a temporary rise that returned to normal within days. No long term safety problems have been reported so far.
Monitoring Safety and Understanding the Genetic Impact
Because CRISPR editing permanently changes DNA inside cells, regulators require many years of safety follow up. Every person in the trial will be monitored for at least one year in the main study, with a required 15 years of long term safety checks. Early signs have been encouraging. No dose limiting toxic effects occurred in the study. Researchers did report two serious health events in the months after treatment, including a spinal disk herniation in one person and a sudden death in another. Neither was considered related to the therapy.
Scientists also measured how strongly CTX310 switched off the ANGPTL3 gene. Higher doses produced much larger reductions, with the 0.7 mg and 0.8 mg doses cutting ANGPTL3 levels by more than 70 percent on average. That drop aligned with the large reductions seen in LDL cholesterol and triglycerides.
What Comes Next for This Experimental Therapy
The Phase 1 trial was designed to test basic safety and early effectiveness. Because it included only 15 people, most of whom were men, the results cannot yet be applied to the broader population. The group also had several different lipid disorders, which makes it harder to predict how the treatment would work for each condition on its own. Larger Phase 2 studies are planned to begin in late 2025 or early 2026. Those trials will enroll more diverse patient groups, follow participants longer and measure how well the treatment protects against real world heart and vascular events.
Researchers believe this early success hints at a future where people with lifelong lipid disorders may not need daily or monthly treatment. A single, lasting change made early enough might prevent decades of risk. Stephen J. Nicholls, lead study investigator and director of the Victorian Heart Institute at Monash University, said the study offered a rare chance to run a pivotal first in human gene editing trial in the region.
Practical Implications of the Research
If larger studies confirm the safety and long lasting effect of CTX310, people living with severe cholesterol or triglyceride problems could benefit from a treatment that frees them from daily pills or repeated injections.
A reliable one time therapy could help people who struggle to stay on medication and reduce the risk of early heart attacks and strokes. It may also set the stage for future gene editing treatments aimed at chronic diseases that require lifelong care.
Researchers hope that if these benefits hold, this approach could reshape heart disease prevention and add another powerful tool to reduce one of the world’s leading causes of death.
Research findings are available online in the journal The New England Journal of Medicine.
Related Stories
- New drug reduces both cholesterol levels and body weight, helping millions
- Cholesterol-lowering drugs help combat Alzheimer's disease
- Common cholesterol drugs could stop cancer growth
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joseph Shavit
Writer, Editor-At-Large and Publisher
Joseph Shavit, based in Los Angeles, is a seasoned science journalist, editor and co-founder of The Brighter Side of News, where he transforms complex discoveries into clear, engaging stories for general readers. With vast experience at major media groups like Times Mirror and Tribune, he writes with both authority and curiosity. His writing focuses on space science, planetary science, quantum mechanics, geology. Known for linking breakthroughs to real-world markets, he highlights how research transitions into products and industries that shape daily life.