First-of-its-kind gene therapy shows promise in shrinking liver cancer tumors
Researchers have demonstrated the potential of gene therapy to effectively shrink hepatocellular carcinoma, a deadly form of liver cancer
[Dec. 2, 2023: JJ Shavit, The Brighter Side of News]
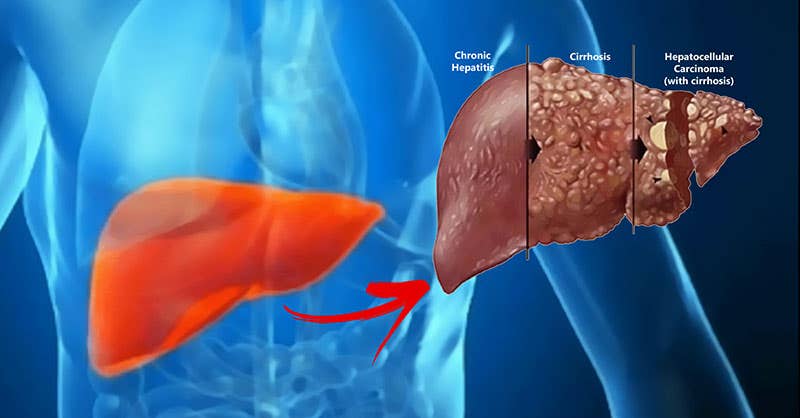
Researchers have demonstrated the potential of gene therapy to effectively shrink hepatocellular carcinoma (HCC), a prevalent and often deadly form of liver cancer. (CREDIT: Creative Commons)
In a groundbreaking study, researchers at UC Davis Comprehensive Cancer Center have demonstrated the potential of gene therapy to effectively shrink hepatocellular carcinoma (HCC), a prevalent and often deadly form of liver cancer.
By targeting the galectin 1 (Gal1) protein, which is frequently overexpressed in HCC, the team not only reduced tumor size but also bolstered the anti-cancer immune response within the tumors, offering hope for improved treatments in the future. The findings of this study, published in Acta Pharmaceutica Sinica B, hold significant promise for HCC patients and potentially those with other types of cancer.
HCC, a liver cancer that originates in the liver cells, has seen a worrisome increase in incidence over the past few decades, making it one of the most common cancers worldwide. Unfortunately, its advanced stages are often associated with a dismal five-year survival rate of less than 20%. Led by senior author Yu-Jui Yvonne Wan, a distinguished professor in the Department of Pathology and Laboratory Medicine, this study aims to provide new insights into potential therapeutic strategies for combating this relentless disease.
Hepatocellular carcinoma (HCC), a prevalent and often deadly form of liver cancer. (CREDIT: State of the Art Imaging)
For years, Gal1 has been recognized as a biomarker for HCC, with its levels significantly elevated in liver cancer patients. The team's research now suggests that Gal1 could be more than just a diagnostic marker; it could serve as a promising therapeutic target for treating HCC.
Gal1's Role in HCC Progression
The Gal1 protein plays a crucial role in regulating the immune system's response to foreign invaders. In healthy individuals, it helps prevent the immune system from mistakenly attacking healthy tissues. However, when Gal1 is overexpressed within HCC, it takes on a sinister role, promoting cancer growth and preventing the immune system from recognizing and attacking the tumor. Notably, the study found a direct correlation between elevated Gal1 levels and aggressive disease progression, as well as poor survival outcomes, mirroring the observations in actual HCC patients.
Related Stories:
Earlier research conducted by the Wan lab utilized gene therapy to elevate the levels of microRNA-22 (miR-22), a non-coding RNA that plays a key role in regulating gene expression. This approach demonstrated that miR-22 overexpression could effectively reduce liver inflammation, treat HCC, and improve survival outcomes when compared to the FDA-approved HCC drug, Lenvatinib, in animal models.
Interestingly, miR-22 was found to downregulate the activity of several genes, including Gal1. This discovery prompted the researchers to explore whether reducing Gal1 levels could be a crucial mechanism by which miR-22 combats HCC.
Inhibiting Gal1 with Gene Therapy
To inhibit Gal1, the research team employed a short interfering RNA (siRNA) known as Igals1. This siRNA was encapsulated within adeno-associated virus 9, a delivery system that exhibits a preference for liver tissue. Once delivered to the tumor site, Igals1 effectively silenced Gal1 in both the cancerous cells and the surrounding stromal tissues that support the tumor's growth.
Graphical abstract: Targeting Gal1 is effective in preventing as well as treatment of HCC and has translational potential. (CREDIT: Acta Pharmaceutica Sinica B)
The results were promising. Silencing Gal1 led to a significant reduction in HCC tumor size, a noteworthy achievement given the stubborn resistance of these tumors to conventional therapies. Furthermore, the therapy succeeded in reducing Gal1 expression within the stroma surrounding the tumor, thereby impacting the tumor microenvironment itself.
A New Avenue for Cancer Treatment
While adeno-associated virus-based gene therapies have been approved by the FDA for the treatment of genetic disorders like spinal muscular atrophy and Hemophilia B, their application in cancer treatment has remained relatively unexplored. The results of this study, along with other ongoing research, demonstrate the potential of gene therapy as a viable avenue for developing innovative cancer treatments.
Gal1 is overexpressed in human and mouse HCC. mRNA levels of LGALS1 in tumors (n = 11) and histological normal livers (n = 9) of HCC patients. Among them, six tumors and adjacent normal tissues were paired, i.e., derived from the same patients. (CREDIT: Acta Pharmaceutica Sinica B)
The implications of this research extend beyond HCC. Gal1 is known to be overexpressed in various other cancer types, including breast, colon, and lung cancers. Additionally, the protein's accumulation begins in diseased livers long before the development of HCC, suggesting that Gal1 inhibition could be considered as a preventive measure against liver cancer.
Lead author Tahereh Setayesh, formerly a post-doctoral researcher in Wan's lab and currently at the Cincinnati Children's Hospital Medical Center, emphasized the significance of this approach, stating, "Silencing galectin 1 is potentially a groundbreaking strategy in the fight against liver cancer. It offers the potential to treat HCC, as well as holding the promise of prevention, providing a path towards transformative therapies."
As the fight against cancer continues, this study represents a significant step forward in the quest for more effective treatments and ultimately, a cure.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.



