New combination drug therapy effectively treats chronic wound infections
Researchers have uncovered ways to make P. aeruginosa vulnerable again, restoring the power of long-standing antibiotics that had stopped working.
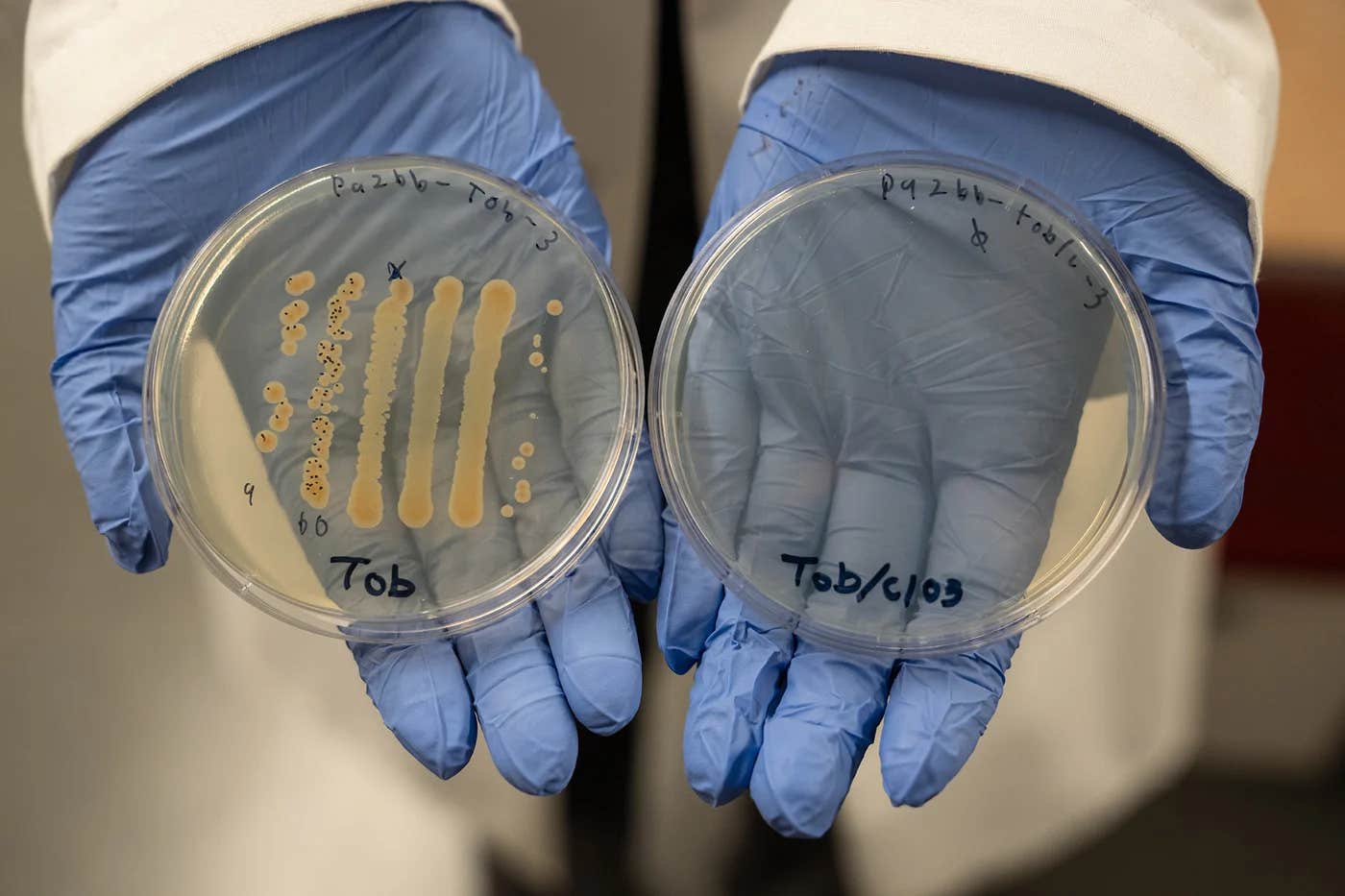
Researchers at the University of Oregon found that pairing chlorate with existing antibiotics amplified their effectiveness against bacteria 10,000-fold compared to single-drug antibiotics. (CREDIT: University of Oregon)
Few hospital-acquired infections are as antibiotic-resistant as Pseudomonas aeruginosa. This opportunistic germ has earned a reputation for beating nearly every drug in the doctor's arsenal. It thrives where oxygen is scarce — in deep, chronic wounds, cystic fibrosis lungs, or even on the surfaces of medical implants — and protects itself in impenetrable biofilms that are resistant to drugs. But a new discovery brings hope by using the microbe's own survival tactics against it.
Instead of developing a new breed of antibiotics, scientists are learning how to take over bacterial metabolism — the process that keeps such cells living in low-oxygen environments. Teams led by researchers like Gentry, Lear, and University of Oregon biologist Melanie Spero found ways to make P. aeruginosa susceptible again and revive the old antibiotics' potency.
Finding Weakness in a Strength
The secret lies in the manner in which the bacteria breathe. In the absence of oxygen, P. aeruginosa uses anaerobic respiration. It relies on chemicals like nitrate and nitrite instead of oxygen to keep energy pumping through its cells. That adaptation slows the growth of the microbe and enables it to weather the storm against antibiotics — the majority of them work best against quickly dividing bacteria.
Gentry and Lear's scientists discovered that interrupting this standby energy source makes the bacteria's weakest link exploitable. By inhibiting enzymes that help convert nitrate into nitrogen gas — a process with multiple steps, known as denitrification — the scientists cut off the bacteria's energy supply. That interference re-sensitized them to standard antibiotics like tobramycin and ciprofloxacin.
The effect was dramatic. Antibiotics that had not worked before began killing bacteria again. It was as if cutting the microbe's power source made it susceptible to attacks that previously bounced off.
Reawakening Old Antibiotics
In Spero's lab at the University of Oregon, his researchers carried this concept further. In their recently published article in Applied and Environmental Microbiology, they revealed a simple chemical trick that boosted antibiotic efficacy 10,000-fold in the laboratory flask. With small doses of chlorate — an inhibitor molecule for enzymes — mixed with currently available antibiotics, the researchers eliminated P. aeruginosa populations that were resistant to monotherapy.
Chlorate, nontoxic to humans at the levels used, was the drug activity-catalyzing agent. "It stressed the bacterial cell so that it's just incredibly susceptible to antibiotics," Spero said. Her researchers could even mix up a combination that was so effective that they could use one percent of a typical dose of antibiotic and still achieve the same effect as killing.
That synergy level could transform the way chronic infections are treated, especially for diabetic foot wounds and other non-healing wounds. Such low-oxygen environments provide perfect hideaways for P. aeruginosa, where traditional treatments fail. "Anything we can do to minimize how long a person is going to be taking antibiotics and minimize the dose, the better," Spero said.
Why Chronic Infections Resist Treatment
Chronic wounds — open sores that heal in weeks or months — create oxygen-poor environments that hinder healing. For diabetics, about one in four develop a foot ulcer, and over half of those become infected. The most severe become amputations.
The issue is that P. aeruginosa does not need oxygen in order to survive. It adjusts its metabolism to operate using nitrate as an alternate source of energy, allowing it to survive despite the body's immune reaction or the administration of antibiotics. Traditional antibiotics, designed for aerobic conditions in experimental laboratory models, don't work since they cannot penetrate through this low-oxygen level.
But when chlorate is added, it appropriates the same nitrate respiration pathway the bacteria depend on. Instead of fueling energy production, chlorate disrupts it, rendering the cells weakened and vulnerable. Spero's earlier work at Caltech proved that this technique not only worked in cell cultures but also in diabetic mouse models, where the combination therapy eradicated infections aggressively.
A Closer Look Inside the Cell
To observe why this pair works so well, researchers examined how the regulatory network of the bacteria responds to low oxygen levels. When oxygen is scarce, a master switch called Anr activates genes that allow the microbe to survive without oxygen. To disable this switch is to send the bacteria into an energy crisis.
When the cell cannot maintain its energy gradient — alternatively, its proton motive force — critical processes start to come to an end. Efflux pumps, where bacteria expel antibiotics, fail to function. Nutrient uptake comes to a standstill. And toxic intermediates like nitric oxide build up, killing the microbe from within outward. In effect, the bacteria start dismantling themselves.
Experiments confirmed this chain reaction. With oxygen-limited conditions, blocking nitrate respiration caused disastrous cell death, even in mature biofilms that are otherwise treatment-resistant. It was a vivid demonstration of how a change in metabolism could turn bacterial survival tactics against them as fatal vulnerabilities.
Opening a New Front in the War on Resistance
Antibiotic resistance is one of the biggest challenges facing modern medicine. According to the World Health Organization, P. aeruginosa is one of the world's most perilous pathogens — one that desperately requires new answers. It takes a long and costly process to develop new antibiotics, but disrupting bacterial metabolism provides a bypass.
"Finding instances of synergy between current antimicrobials is going to be very valuable," Spero said. Rather than coming up with new medicines, doctors may combine and recombine current ones with metabolic inhibitors to create new therapies.
This concept, also known as "metabolic adjuvant therapy," could extend well beyond P. aeruginosa. Many pathogens take the same anaerobic route when oxygen is in short supply — including urinary tract infection-causing and pneumonia-causing pathogens. If metabolic inhibitors can be made clinic-safe, they could revive the potency of dozens of antibiotics.
From the Lab to the Clinic
The second challenge is turning these findings into actual treatments. Chlorate and the related compounds will have to undergo strict safety testing before they can be tried in humans. Scientists also need to observe the drug combinations in mixed microbial communities, as chronic wounds tend to harbor many different species cohabiting together.
Spero's laboratory, with the help of a $1.84 million National Institutes of Health grant, is striving to understand how these chemical mixtures work in complex microbial communities. Eventually, the researchers hope to understand which cellular stress pathways make bacteria vulnerable so that researchers can design new synergistic drugs with precision rather than trial and error.
For now, the findings are a breath of fresh air in the struggle to regain antibiotics that had become ineffective. By learning about and taking advantage of the adaptive biology of bacteria, researchers are showing that even the most resistant microbes have weaknesses — if you know where to look.
Practical Implications of the Research
If this metabolic hijacking approach works in a clinical scenario, it would transform the management of chronic infections. Patients would spend fewer days on antibiotics, fewer toxic side effects, and fewer complications like amputations from chronic wounds. Hospitals would be able to better contain infections that thrive on medical devices or ventilators, where there is low oxygen.
More broadly, the findings suggest a new tack for the global fight against antibiotic resistance — not through constant innovation, but through more astute use of what medicine already has at hand.
By pairing antibiotics with medications that neutralize bacterial defenses, doctors may be able to revive old drugs, giving humanity a stronger hold on its ongoing fight against superbugs.
Research findings are available online in the journal Applied and Environmental Microbiology.
Related Stories
- Tiny nanoparticles and vinegar team up to fight deadly wound infections
- New bio-inspired medical glue seals bleeding wounds in seconds
- Bee-stinger microneedle patch speeds wound healing with smart monitoring
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Rebecca Shavit
Writer
Based in Los Angeles, Rebecca Shavit is a dedicated science and technology journalist who writes for The Brighter Side of News, an online publication committed to highlighting positive and transformative stories from around the world. Her reporting spans a wide range of topics, from cutting-edge medical breakthroughs to historical discoveries and innovations. With a keen ability to translate complex concepts into engaging and accessible stories, she makes science and innovation relatable to a broad audience.



