Scientists build tiny biological robots from human cells to drive regenerative healing
In the future, patient-derived biobots could serve as therapeutic tools for regeneration, healing, and disease treatment.
[Dec. 2, 2023: JD Shavit, The Brighter Side of News]
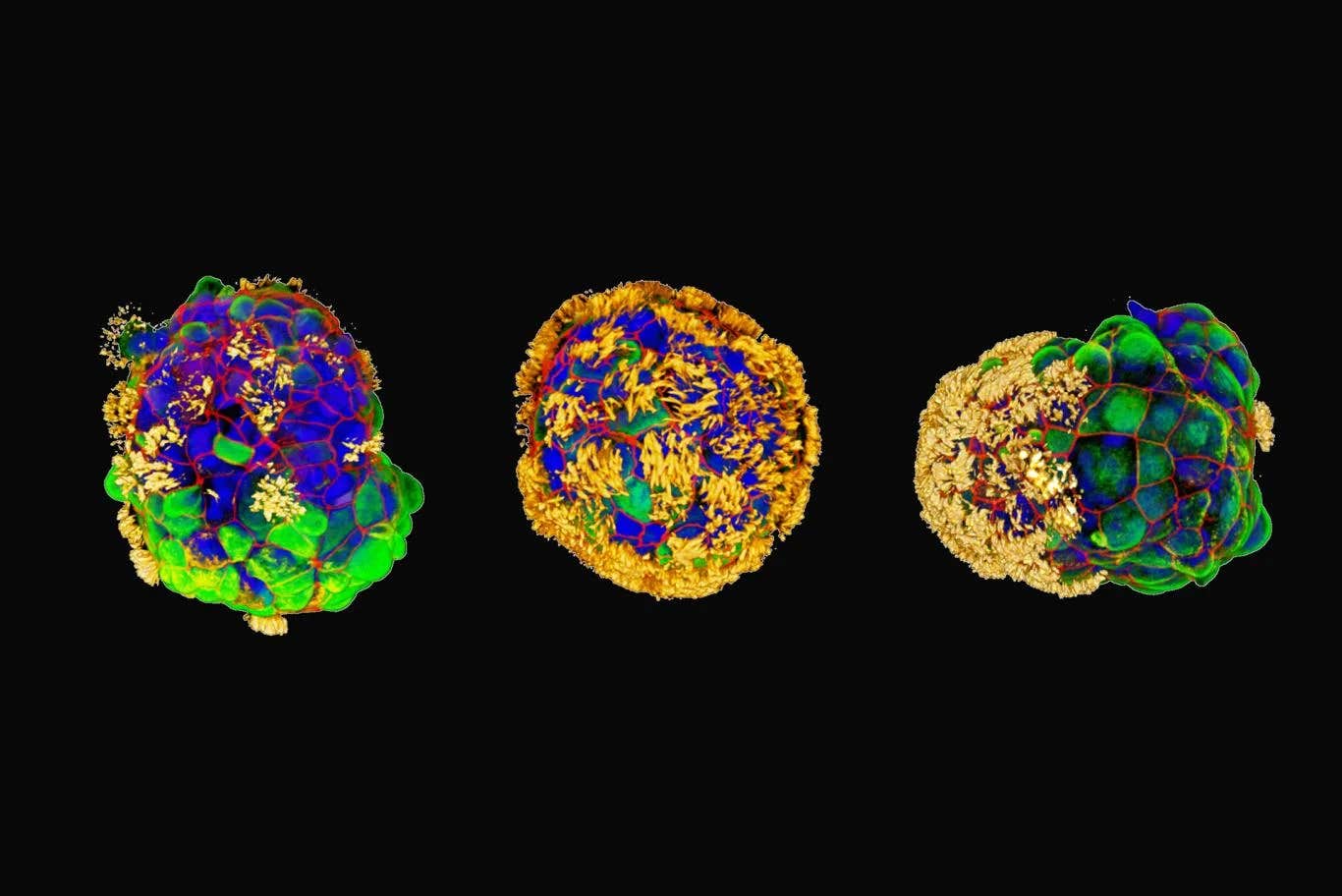
Three examples of Anthrobots with hair-like cilia in yellow. “It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move on their own and encourage neuron growth across a region of damage,” said Michael Levin. (CREDIT: Gizem Gumuskaya)
In a groundbreaking discovery, researchers from Tufts University and Harvard University's Wyss Institute have unleashed the potential of tiny biological robots known as "Anthrobots," created from human tracheal cells. These remarkable multicellular entities, ranging in size from the width of a human hair to the tip of a sharpened pencil, have not only demonstrated the ability to self-assemble but have also exhibited a profound healing effect on neighboring cells. This transformative research offers a glimpse into a future where patient-derived biobots could serve as therapeutic tools for regeneration, healing, and disease treatment.
The genesis of this extraordinary achievement traces its roots to earlier work conducted in the laboratories of Michael Levin, the Vannevar Bush Professor of Biology at Tufts University School of Arts & Sciences, and Josh Bongard at the University of Vermont. These pioneering researchers had previously engineered multicellular biological robots, known as Xenobots, using frog embryo cells. These Xenobots showcased a range of capabilities, from navigating passageways and collecting materials to self-healing and limited replication.
However, a lingering question persisted: were these capabilities exclusive to amphibian embryos, or could biobots be fashioned from cells of other species? The answer to this inquiry would emerge in the form of the Anthrobots, a novel creation with unprecedented potential.
In a study published in Advanced Science, Michael Levin, alongside PhD student Gizem Gumuskaya, unveiled the revelation that Anthrobots could indeed be fashioned from adult human cells without the need for genetic modification. This discovery marks a significant step toward unraveling the mysteries of how cells assemble and cooperate within the human body, and how they can be reconfigured into alternative "body plans" for specialized functions.
Related Stories
Gumuskaya, who boasts a background in architecture, elaborated on the research's essence: "We wanted to probe what cells can do besides create default features in the body. By reprogramming interactions between cells, new multicellular structures can be created, analogous to the way stone and brick can be arranged into different structural elements like walls, archways, or columns."
The researchers observed that not only could these cells create new multicellular shapes, but they also exhibited unique locomotion capabilities when placed on a surface with human neurons in a lab dish. Furthermore, they had the intriguing ability to stimulate the growth of neurons, effectively bridging gaps caused by damage to the neural layer.
The mechanism behind how Anthrobots encourage neural growth remains a subject of ongoing investigation, but their potential as healing agents is undeniable. Levin expressed his fascination with the discovery, stating, "It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move on their own and encourage neuron growth across a region of damage. We're now looking at how the healing mechanism works, and asking what else these constructs can do."
An Anthrobot is shown, depth colored, with a corona of cilia that provides locomotion for the bot. (CREDIT: Gizem Gumuskaya, Tufts University)
The use of human cells offers a range of advantages, including the ability to construct biobots from a patient's own cells without triggering an immune response or necessitating immunosuppressants. Anthrobots have a limited lifespan, breaking down after a few weeks and easily reintegrating into the body once their work is completed.
Moreover, Anthrobots can only survive in controlled laboratory conditions, eliminating the risk of unintended exposure or spread outside the lab. They do not possess the capacity for reproduction, nor do they undergo any genetic alterations, ensuring that they remain within established safety boundaries.
The Anthrobot Creation Process
The birth of each Anthrobot begins with a single cell, obtained from an adult donor's tracheal surface. These cells are equipped with cilia, hair-like projections that perform the crucial function of expelling foreign particles and excess fluids from the air passages of the lungs. By cultivating these cells in a lab setting, researchers observed that they spontaneously formed multicellular spheres, termed organoids.
An aggregate of Anthrobots, or superbot (green), stimulates growth of neurons (red) where they had been mechanically stripped away. (CREDIT: Gizem Gumuskaya, Tufts University)
The researchers refined their growth conditions to encourage the outward-facing orientation of cilia on these organoids. Within a few days, the organoids began exhibiting autonomous movement, driven by cilia that functioned akin to oars. This marked the first significant feature of the biobot platform. Levin proposed that by incorporating additional features into the Anthrobots, such as those contributed by different cell types, they could be programmed to respond to their environment, travel within the body, or participate in the construction of engineered tissues in the lab.
The research team, in collaboration with Simon Garnier at the New Jersey Institute of Technology, categorized the various types of Anthrobots produced. These bots fell into distinct categories of shape and movement, with sizes ranging from 30 to 500 micrometers—filling a vital niche between nanotechnology and larger engineered devices.
Some Anthrobots took on spherical shapes, adorned with cilia covering their entire surface, while others exhibited irregular or football-shaped forms with sporadic cilia coverage or cilia present on one side only. Their movement patterns included straight-line trajectories, tight circular motions, a combination of both, or even stationary wiggling. Typically, these creations endured laboratory conditions for approximately 45 to 60 days before undergoing natural biodegradation.
Gumuskaya emphasized the convenience of Anthrobot self-assembly, stating, "Anthrobots self-assemble in the lab dish. Unlike Xenobots, they don't require tweezers or scalpels to give them shape, and we can use adult cells—even cells from elderly patients—instead of embryonic cells. It's fully scalable—we can produce swarms of these bots in parallel, which is a good start for developing a therapeutic tool."
Little Healers
With an eye toward therapeutic applications, Levin and Gumuskaya designed a laboratory test to assess how Anthrobots could aid in wound healing. The experiment involved cultivating a two-dimensional layer of human neurons and creating an open "wound" by gently scratching the cell layer with a thin metal rod.
To ensure that the wound area was exposed to a dense concentration of Anthrobots, the researchers engineered "superbots"—clusters naturally formed when Anthrobots are confined to a restricted space. Comprising primarily circlers and wigglers, these superbots were strategically designed to remain in proximity to the open wound.
Surprisingly, the unmodified Anthrobots triggered substantial neural regrowth, forming a bridge of neurons as thick as the surrounding healthy cells on the plate. In areas devoid of Anthrobots, neurons did not exhibit any regrowth. Within the simplified 2D environment of the lab dish, Anthrobot assemblies demonstrated remarkable efficacy in facilitating the healing of live neural tissue.
The implications of this discovery are vast. The development of Anthrobots holds the potential to revolutionize various applications, including clearing plaque buildup in the arteries of atherosclerosis patients, repairing spinal cord or retinal nerve damage, identifying bacteria or cancer cells, and delivering targeted drug therapies. Anthrobots could play a pivotal role in tissue regeneration while dispensing pro-regenerative drugs.
Making New Blueprints, Restoring Old Ones
Gumuskaya illuminated the innate capabilities of cells to self-assemble into larger structures, describing fundamental processes: "The cells can form layers, fold, make spheres, sort and separate themselves by type, fuse together, or even move. Two important differences from inanimate bricks are that cells can communicate with each other and create these structures dynamically, and each cell is programmed with many functions, like movement, secretion of molecules, detection of signals and more. We are just figuring out how to combine these elements to create new biological body plans and functions—different than those found in nature."
Human bronchial epithelial cells self-construct into multicellular motile living architectures. (CREDIT: Advanced Science)
This approach harnesses the inherent flexibility of cellular assembly, not only for constructing Anthrobots but also for gaining insights into natural body plan formation. It sheds light on the intricate interplay between the genome and the environment in creating tissues, organs, and limbs, offering hope for the restoration of these structures through regenerative therapies.
The journey of Anthrobots from tracheal cells to multifunctional healing agents exemplifies the power of cellular reprogramming and cooperation. While many questions remain, this research has undeniably opened the door to a realm of possibilities in the fields of regenerative medicine, biotechnology, and beyond. The Anthrobots represent a remarkable convergence of biology and robotics, pointing the way toward a future where the body's own cells can be harnessed to heal and restore, pioneering new horizons in the quest for health and well-being.
For more science and technology news stories check out our New Innovations section at The Brighter Side of News.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.



