Scientists recharge and repair human cells using donated mitochondria
New study at Texas A&M University shows how boosted stem cells can donate energy units to repair and recharge damaged human cells.

 Edited By: Joseph Shavit
Edited By: Joseph Shavit
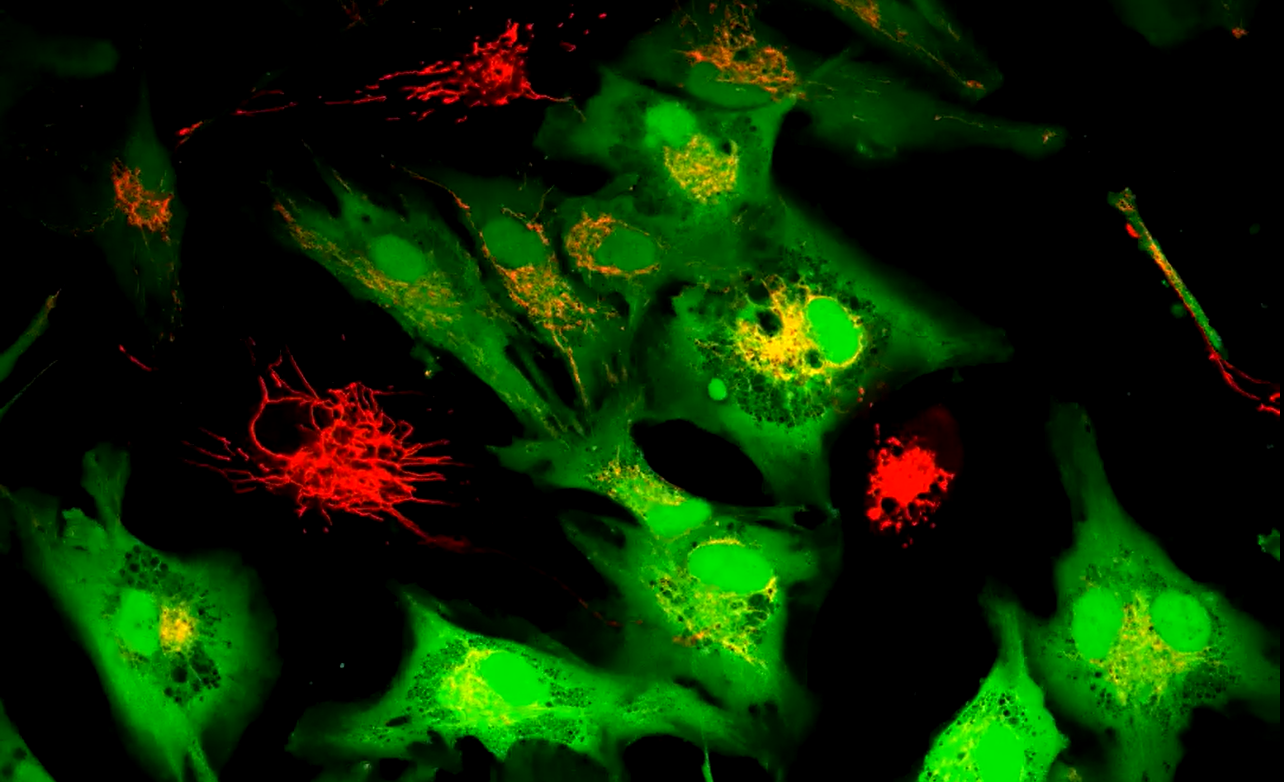
Researchers boosted stem cells to share extra mitochondria with failing cells, restoring energy and cutting damage in lab tests. (CREDIT: PNAS)
Tiny structures inside your cells keep your body alive by turning food into fuel. These structures, called mitochondria, power every heartbeat, thought, and movement. When they fail, organs that need the most energy suffer first, including the heart, brain, and muscles. Many serious diseases trace back to this breakdown, yet most treatments today only ease symptoms. They do not fix the energy problem itself.
Now, scientists say a new method could change that. A team at Texas A&M University has found a way to help healthy cells donate extra mitochondria to damaged neighbors. The research, published in PNAS, shows a path toward restoring power where cells have gone dark.
A Natural Repair System, Supercharged
Cells already share mitochondria in small amounts. When one cell is stressed, another can send help. Stem cells are especially good at this rescue work. The trouble is that the process happens too rarely to serve as a treatment. The researchers asked a simple question: What if donor cells had more power to give?
To answer it, they used tiny, flower-shaped particles made from a compound called molybdenum disulfide. These “nanoflowers” act like sponges inside cells. They soak up harmful oxygen molecules that build up under stress. When those molecules drop, a chain reaction begins inside the cell that leads to more mitochondria growth.
In lab tests, stem cells treated with the nanoflowers doubled their mitochondrial DNA in just seven days. That signaled a major rise in the number of working power units inside each cell. Best of all, the process did not rely on drugs or genetic changes.
“We have trained healthy cells to share their spare batteries with weaker ones,” says biomedical engineer Akhilesh Gaharwar. “By increasing the number of mitochondria inside donor cells, we can help aging or damaged cells regain their vitality, without any genetic modification or drugs.”
How the Boost Works
The nanoflowers reduce harmful molecules called reactive oxygen species. That shift activates a protective enzyme known as SIRT1. In turn, SIRT1 flips on a master switch for energy growth called PGC-1α. Once this switch is on, the cell starts building new mitochondria at a fast pace.
The team tested particles from about 100 to 250 nanometers wide. The smallest ones slipped into cells more easily and worked at lower doses. At safe levels, the particles caused no harm to growth or health.
Passing Power Through Cell Tunnels
Next, the scientists watched what happened when these supercharged stem cells met injured cells. They placed the cells side by side and waited.
Within a day, tiny bridges formed between them. Through these tunnels, known as tunneling nanotubes, mitochondria moved from the stem cells into heart cells, muscle cells, and smooth muscle cells.
The results were striking. Compared with normal stem cells, the treated ones delivered about twice as many mitochondria into muscle cells. Transfers into heart-related cells and smooth muscle cells rose by three to four times.
When the team blocked tunnel growth, the transfer stopped. When they separated the cells with filters, the sharing also halted. These checks proved the mitochondria moved through direct contact, not through the fluid around the cells. The particles themselves never crossed into the recipient cells.
Energy Returns
Extra mitochondria matter only if they work. The team measured the cells’ energy supply and found big improvements. Levels of ATP, the cell’s main fuel, climbed. Oxygen use also rose, a sign that the donated mitochondria were active.
The boost did not come from changes in sugar use. The cells were not just eating differently. They were running on new power from fresh mitochondria.
Inside the Cell After the Swap
The scientists also looked at how cells changed on a genetic level. Thousands of genes shifted their activity after the transfer. Many of them controlled energy flow and the upkeep of mitochondria.
Genes inside the mitochondria did not change. Instead, genes in the cell nucleus revved up. That showed the cell’s control center was adapting to support the new arrivals.
Key energy systems grew stronger. The cell began building proteins needed for mitochondria function and assembling the parts that produce ATP.
Healing Damaged Cells
To test real harm, the team exposed cells to toxins known to wreck mitochondria. One chemical blocked energy flow. Another collapsed electrical balance. A third was doxorubicin, a chemotherapy drug that often harms the heart.
In every case, the donated mitochondria helped reverse the damage. ATP levels returned near normal. Harmful molecules fell. Electrical balance improved. Fewer cells died.
Heart cells damaged by chemotherapy showed the greatest recovery. Signals tied to programmed cell death dropped, while healthy energy use returned. It was a clear sign that the rescue had worked.
From Lab Dish to Living Patients
The idea is simple: deliver energy, not just medicine. Instead of managing symptoms, future care could treat the root cause by sending power where it is needed.
Geneticist John Soukar calls the results encouraging. “It’s pretty promising in terms of being able to be used for a whole wide variety of cases, and this is just kind of the start,” he says. “We could work on this forever and find new things and new disease treatments every day.”
Possible uses range from heart disease to muscle loss. Treatment could involve preparing stem cells with nanoflowers before transplant or directing therapy to weak tissues. The particles can be modified to aim at specific organs, such as the heart or brain.
Still, the team stresses caution. Animal tests are ongoing. Studies in people will take time. Researchers need to learn how best to deliver the cells, what doses are safe, and how long the benefits last.
“This is an early but exciting step toward recharging aging tissues using their own biological machinery,” Gaharwar says. “If we can safely boost this natural power-sharing system, it could one day help slow or even reverse some effects of cellular aging.”
For now, the work remains in the lab. But for diseases tied to energy failure, it offers something rare: a chance to fix the engine, not just the warning light.
Practical Implications of the Research
This research could open new treatment paths for heart disease, muscle disorders, and conditions linked to aging and low energy in cells. By restoring power inside sick cells, doctors may one day slow damage instead of only easing pain or loss of function.
The approach could also reduce side effects from drugs that harm the heart during cancer treatment. In the long term, it may lead to therapies that help tissues heal themselves by sharing healthy mitochondria.
Research findings are available online in the journal PNAS.
Related Stories
- Space travel accelerates stem cell aging - threatening astronaut health
- New drug Rapalink-1 slows cellular aging, study finds
- New protein discovery opens the door to slowing or even reversing cellular aging
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Mac Oliveau
Science & Technology Writer
Mac Oliveau is a Los Angeles–based science and technology journalist for The Brighter Side of News, an online publication focused on uplifting, transformative stories from around the globe. Passionate about spotlighting groundbreaking discoveries and innovations, Mac covers a broad spectrum of topics—from medical breakthroughs and artificial intelligence to green tech and archeology. With a talent for making complex science clear and compelling, they connect readers to the advancements shaping a brighter, more hopeful future.