Single protein is key to treating a deadly genetic heart disease targeting young athletes
UC San Diego scientists report that restoring connexin-43 dramatically improves heart function and survival in arrhythmogenic cardiomyopathy.

 Edited By: Joseph Shavit
Edited By: Joseph Shavit
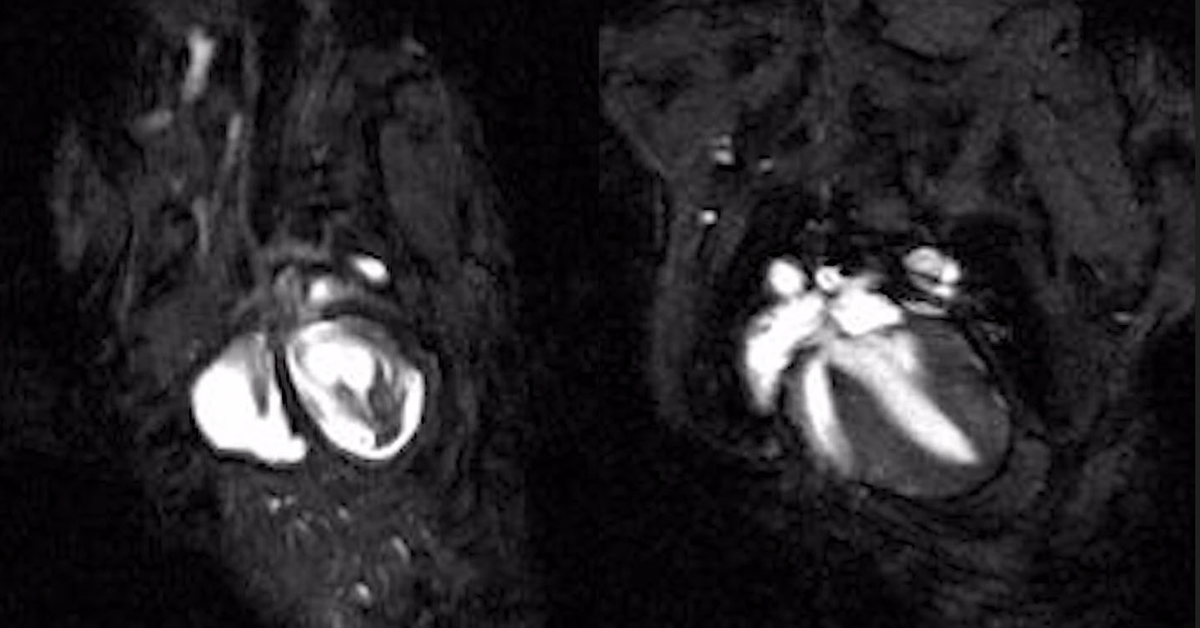
Cardiac magnetic resonance imaging (MRI) videos of beating hearts in an inherited arrhythmogenic cardiomyopathy mouse model, shown untreated (Left) and after connexin-43 gene therapy treatment (Right), illustrating repair of impaired heart function. (CREDIT: UC San Diego Health Sciences)
A research team at the University of California San Diego has discovered a novel and promising method of treating arrhythmogenic cardiomyopathy (ACM), a rare inherited heart disease that can strike suddenly and violently, developing into a life-threatening condition in young, healthy athletes. By restoring expression of an important protein, connexin-43, in murine (mouse) models, the investigators dramatically improved overall heart function, significantly reduced dangerous heart arrhythmias, and increased survival rates by greater than twofold.
Patients with ACM do not have the ability to efficiently pump blood due to damage to their cell-to-cell contact proteins, known as desmosomes, which are weakened by genetic defects. Furthermore, patients with ACM experience an increased risk for sudden cardiac death, particularly athletes, because of the additional stress placed on an already damaged, structurally weak heart by prolonged, high levels of activity.
Results of this study, published in the journal Circulation: Heart Failure, indicate that one potential gene-based therapy will offer hope for the treatment of many different types of patients with this condition. This benefit appears regardless of the underlying etiology of their ACM, despite the many pathogenic variants of the ACM gene.
Clinical Features And Disease Progression
“At first glance, you would think these individuals were in great health because of their ability to perform physical activities, but in reality, they have inherited genetic defects that disrupt the normal desmosome structure in their hearts, thereby allowing for structural weakness of the heart muscles,” said Dr. Farah Sheikh, the senior author of this study and a professor in the Department of Medicine at the UC San Diego School of Medicine.
Eventually, as the cells fail, scar tissue replaces heart muscle cells. “Once heart muscle cells begin to fail, the heart will become increasingly susceptible to mechanical stress associated with each heartbeat. This process eventually leads to sudden cardiac death or, in some cases, to heart failure,” Sheikh said.
The cause of ACM is due to histopathologic genetic mutations of desmosome genes, which cause dysfunction of the heart muscle. As a result, when desmosomal gene mutations occur, the strength and electrical stability of the heart are affected.
Challenges In Treating ACM At The Cellular Level
While different patients may have different mutations in various desmosome genes, it has proved difficult to treat this disorder on a cellular level. Researchers associated with Sheikh’s lab established a gene therapy to target the most typical genetic cause of ACM, which is the plakophilin-2 gene. This treatment is currently being evaluated in initial clinical studies.
However, researchers have identified that certain desmosome genes are still too large to repair with currently available gene therapy technologies. This limitation has made alternative treatment approaches necessary.
In this study, researchers adopted a different treatment approach using connexin-43 as a primary target. The research team found that connexin-43 is an absent or decreased protein expressed in the heart muscle cells of patients with all forms of ACM.
Restoring Connexin-43 To Stabilize Heart Function
Furthermore, connexin-43 allows the conduction of electrical impulses to be transmitted from one heart cell to another. Thus, connexin-43 provides the additional therapeutic benefit of stabilizing the mechanical, or structural, integrity of the heart.
"Using adeno-associated viral vector gene therapy, our research team successfully restored connexin-43 in animal models with severe ACM genetic mutations. These included desmoplakin deficiency and widespread human plakophilin-2 mutations. The therapeutic effect was significant," Sheikh told The Brighter Side of News.
"Treated mice were found to live more than twice as long as untreated control mice. Treated animals also demonstrated enhanced efficiency in blood pumping and avoided developing an enlarged heart due to the advanced stage of disease," she continued.
Structural And Electrical Improvements Observed
Irregular apical heart rate episodes were reduced significantly, and improvements were noted in electrical signaling. Desmosomal proteins that bind the three-dimensional “brick-and-mortar” cellular architecture of the heart back into a normal range were also regenerated through therapy.
Structural defects in the heart were properly corrected despite initiation of therapy in the later stages of disease. The most surprising finding to researchers was that the electrical “repairs” also improved the mechanical integrity of the heart.
The research team used an in vitro human heart muscle cell model generated from induced pluripotent stem cells derived from ACM patients. The in vitro structure of these cells reflected the presence of mutated plakophilin-2 and desmoglein-2.
Connexin-43 And Its Unexpected Role In The Cell Nucleus
The regenerated connexin-43 resulted in stabilization of cells, improvement of beat patterns, and production of desmosomal proteins required to establish strong connections. Further study of connexin-43 revealed an additional and unexpected function.
“Surprisingly, connexin-43 was found to migrate into the nucleus,” explained Sheikh. “This unexpected finding suggests that connexin-43 also has a role in reprogramming heart muscle cells to rebuild their mechanical connections and improve heart function.”
Connexin-43 may not only play a role in holding cells together electrically, but also structurally. Evidence collected during the study indicated that connexin-43 plays an important role in switching on and delivering genes to their respective locations at the cell junctions.
Toward Broader Therapeutic Potential
These junctions are where genes are needed for the production of desmosomal proteins. The idea behind a single protein creating such a broad spectrum of benefit may be due to the dual nature of the therapy.
“We discovered that using connexin-43 gene therapy, we could correct defects in the cellular connections in patients with arrhythmogenic cardiomyopathy,” stated Sheikh. “That leads us to believe this approach may be generally more effective in treating multiple genetic forms of the disease.”
In addition to being a new treatment option for patients suffering from arrhythmogenic cardiomyopathy, Sheikh described connexin-43 gene therapy as an innovative way to “glue” heart muscle cells back together.
Moving Toward Broader Treatment For Heart Disease
LEXEO Therapeutics acquired the connexin-43 gene therapy program to support commercial development of the drug. As part of this support, LEXEO is conducting additional preclinical studies to evaluate long-term safety and determine the most appropriate method of administration for the gene therapy.
Connexin-43 is reduced in other types of cardiomyopathy and heart failure, which indicates potential for broader application. “We want to evaluate how broadly this gene therapy can be utilized within heart disease. We also want to understand the time frame for maximum therapeutic benefit,” stated Sheikh.
Human clinical trials are not yet underway. However, these studies generate hope where an effective option has not existed for many years in arrhythmogenic cardiomyopathy.
Practical Applications Of The Research
In summary, this study has created a more comprehensive treatment approach to arrhythmogenic cardiomyopathy by treating shared dysregulation of the downstream defect, rather than targeting individual genetic errors. Connexin-43 gene therapy may make it easier for patients with a broad spectrum of genetic mutations to access effective treatment.
If additional studies show safety and effectiveness of connexin-43 gene therapy in humans, it has the potential to drastically lower the incidence of sudden cardiac death and increase patient quality of life. It may also provide patients with the opportunity to address heart damage before irreversible damage occurs.
The data also provide the opportunity to develop therapies that address other heart diseases caused by failure to produce sufficient cytoplasmic junctions, such as weakened cell connections, that afflict millions worldwide.
Research findings are available online in the journal Circulation.
Related Stories
- Toddler born deaf hears for the first time after groundbreaking gene therapy
- New gene therapy fixes brain disorders related to autism and epilepsy
- Gene therapy restores hearing in deaf children and adults
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.