Powerful new protein effectively delivers cancer-fighting drugs directly into cells
A new discovery shows how a hidden cell protein helps large cancer drugs enter cells more easily, boosting their power and potential.
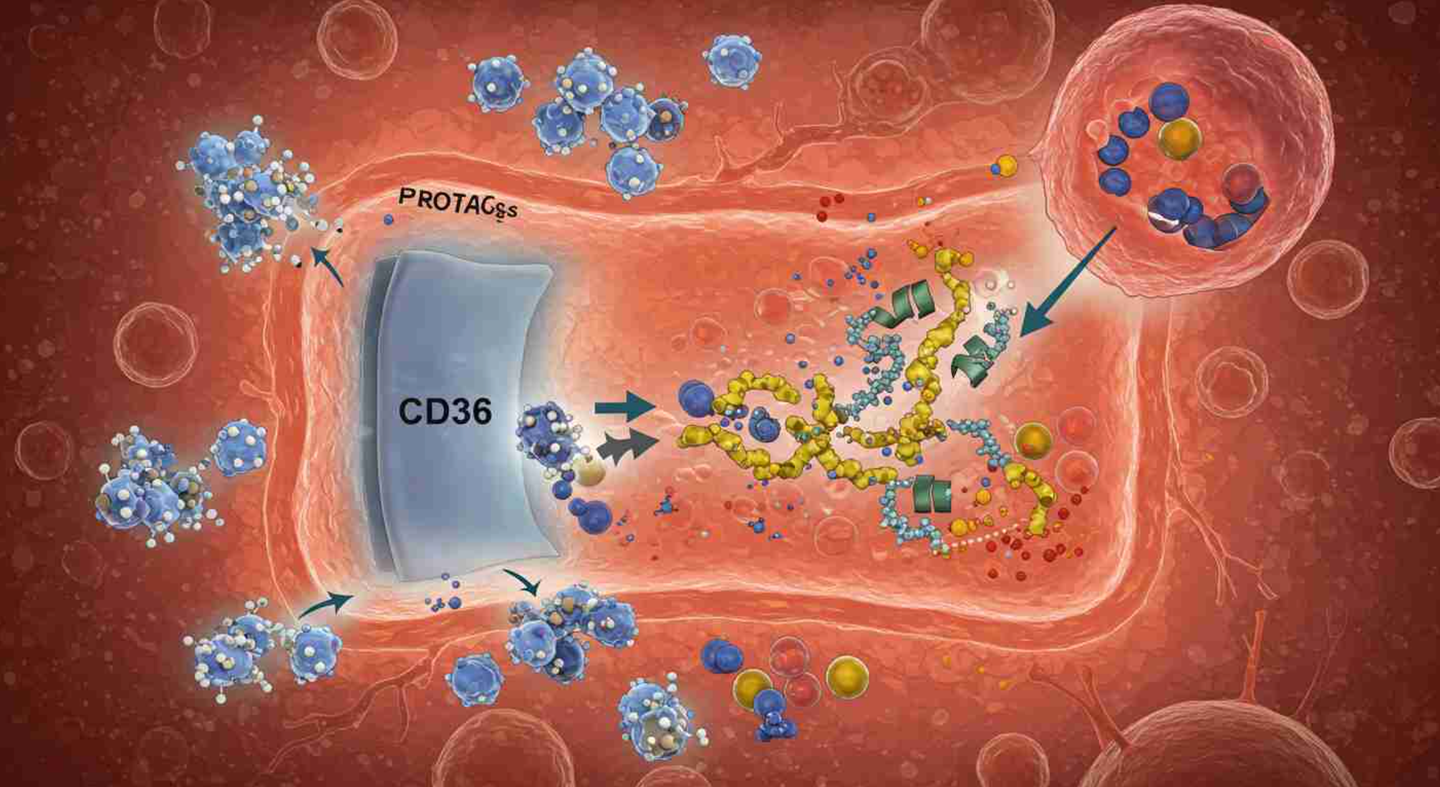
Scientists discover a way to help large cancer drugs enter cells using CD36, boosting treatment power up to 23 times. (CREDIT: CC BY-SA 4.0)
Inside your body, countless processes are happening every second to keep you alive. Among them is a little-known but powerful protein called CD36. Scientists have just discovered that this protein might be the secret to delivering some of the most promising cancer-fighting drugs more effectively into your cells.
This breakthrough could improve how doctors treat cancer and other serious diseases by helping large, complex drugs enter cells more easily.
These special drugs, called PROTACs, are a new type of medicine designed to break down harmful proteins inside your body. Until now, they’ve had one big problem—they’re just too big to get inside cells easily. But researchers found a way to turn that weakness into a strength.
Most medications are small. That’s because they need to pass through your cells' protective outer layer, the cell membrane. If a molecule is too large, it usually can’t squeeze through, and that makes it hard for the drug to do its job. Scientists even have a rule for this: it’s called the "Rule of 5." It says that drugs bigger than 500 daltons—a unit of molecular weight—are generally not effective because they can’t enter cells easily.
But the new PROTAC drugs often weigh over 1,000 daltons. Despite this, they work—and now we’re closer to understanding why.
Researchers from Duke University, the University of Texas at San Antonio, and the University of Arkansas teamed up to explore this puzzle. Published in the journal, Cell, they found that these large drugs can hijack a natural process used by your cells to absorb nutrients: endocytosis. This is when the cell’s outer membrane folds in and "swallows" substances, pulling them inside.
That’s where CD36 comes in. This protein, found on many cell surfaces—especially in your intestines, skin, lungs, and eyes—acts like a gatekeeper. It pulls in large and polar molecules that can’t get through on their own. Scientists used this to their advantage. By designing drugs that attach to CD36, they increased the drug’s absorption by 7.7 to 22.3 times in lab tests. These drugs also became up to 23 times more powerful at killing cancer cells.
“This discovery is important because it could rescue many drugs that were previously considered unusable due to poor absorption,” said Hui-Kuan Lin, PhD, a cancer biology professor involved in the study. “It could turn them into clinically useful treatments.”
Related Stories
Unlike older cancer drugs that simply block parts of proteins, PROTACs destroy them completely. Most cancer drugs focus on stopping just one activity of a harmful protein, usually an enzyme. But proteins often have many roles. Even if one function is blocked, the cancer may still grow and spread. This is one reason cancer can become resistant to treatment.
PROTACs work differently. They bind to a target protein and bring it close to another protein called an E3 ligase. This pair-up leads to the target protein being marked for destruction. The body’s waste-disposal system, the proteasome, then breaks it down completely. That’s what makes PROTACs so promising: they don’t just silence bad proteins—they eliminate them.
“Since PROTACs degrade their target protein and activity, they could reduce the chance of drug resistance,” Lin explained.
These drugs are now being tested for a wide range of conditions, from cancer to Parkinson’s disease. Eight different oral PROTACs are already in clinical trials, with one being studied as a first-line treatment for breast cancer.
Researchers suspected that PROTACs were getting into cells by more than just passive diffusion—the usual way small molecules enter cells. So, they created a special version of a PROTAC drug with a biotin tag, a small molecule often used in labs. This allowed them to identify which proteins it interacted with on the cell surface.
They found that CD36 wasn’t just involved—it was leading the charge. CD36 grabbed onto the drug and helped pull it into the cell. Further testing showed that when CD36 was removed from cancer cells, the drugs lost much of their power. That meant CD36 wasn’t just helping—it was necessary.
Hong-yu Li, PhD, a medicinal chemistry professor at UT San Antonio, was surprised by the results. “For decades, it was thought that molecules this large couldn’t cross membranes effectively,” he said. “Through chemistry and biology, we identified CD36 as a protein for uptake and optimized drugs to better engage with CD36.”
This approach is called chemical endocytic medicinal chemistry (CEMC). Instead of trying to make drugs small enough to sneak in through the membrane, CEMC helps drugs actively enter cells by riding along with proteins like CD36.
Many important drugs don’t follow the Rule of 5. One example is rapamycin, used in cancer therapy and to prevent organ rejection. It’s a large molecule, but it still works. Scientists believe this might also be due to CD36 or similar transport systems.
Tests on mice showed that modified PROTACs using the CD36 pathway led to greater tumor suppression. The drugs didn’t lose their stability or solubility either—two things that can make or break a new medicine.
In prostate and breast cancer cells, removing CD36 blocked the cancer-fighting power of these drugs. This wasn’t just true for PROTACs but for other large drugs too, like doxorubicin and navitoclax. However, smaller drugs like paclitaxel didn’t rely on CD36.
Researchers even looked at how these drugs physically interact with CD36 using a method called surface plasmon resonance. One modified PROTAC, called SIM1-Me, had four times stronger binding to CD36 than palmitic acid, a natural fat that CD36 normally grabs.
Further experiments showed that drugs designed to better latch onto CD36 entered cells more efficiently and broke down harmful proteins faster. This makes CD36 a prime target in future drug development.
This discovery could open doors for treating diseases once considered untreatable. Many conditions involve proteins that were labeled "undruggable" because they couldn’t be targeted with small molecules. PROTACs—and now, the CEMC strategy—change that.
By focusing on how cells take in large molecules, scientists can design more effective and targeted treatments. Instead of forcing drugs to squeeze through the membrane, they can now ride through the front door.
"This was completely unexpected in the research field," said Li. The results of the study were confirmed independently by all three research teams, showing strong evidence that this method works.
Although it’s still early, this strategy holds promise beyond cancer. Diseases involving large or complex proteins—like Alzheimer’s, autoimmune disorders, or infectious diseases—could also benefit.
The next step is testing this method in people. Clinical trials will need to prove that the approach is safe and effective in real patients. But the early data is hopeful.
This discovery is more than just a clever chemical trick—it’s a shift in how we think about treating disease. For decades, scientists tried to design drugs to sneak past the cell wall. Now, they may have found a new, smarter way in.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.